- Home
- »
- Biotechnology
- »
-
Circulating Tumor Cells Market Size & Share Report, 2030GVR Report cover
![Circulating Tumor Cells Market Size, Share & Trends Report]()
Circulating Tumor Cells Market Size, Share & Trends Analysis Report By Application (Clinical/Liquid Biopsy, Research), By Specimen (Bone Marrow, Blood), By Product, By Technology, By End-use, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-287-7
- Number of Pages: 120
- Format: Electronic (PDF)
- Historical Range: 2018 - 2023
- Industry: Healthcare
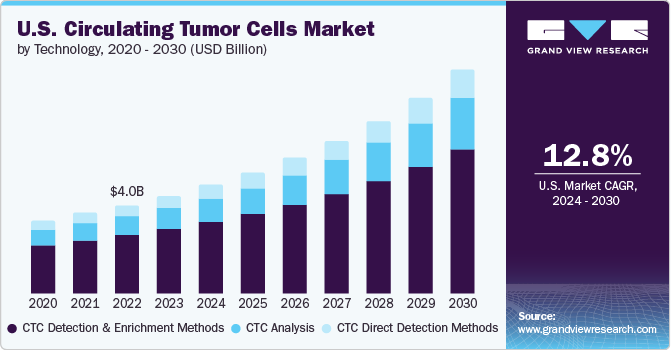
Circulating Tumor Cells Market Size & Trends
The global circulating tumor cells market size was estimated at USD 11.47 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 13.62% from 2024 to 2030. Owing to the non-invasiveness and advantages offered by circulating tumor cells (CTC), it is considered a promising tool in cancer diagnosis. This is anticipated to contribute to the market’s growth. Technological advancements in chip technology is another key factor driving the growth.
The ongoing advancements in chip technology have positively impacted market growth. Cluster chip technology plays a key role in capturing the exceptionally rare group of cells, such as CTCs. Isolation of CTCs is a cumbersome process and requires high accuracy. The chip technology not only helps perform precise CTC isolation but also helps overcome the challenges associated with isolation devices developed by key companies. The challenges include lower sensitivity, the inability to capture CTCs of all sizes & types, high manufacturing costs, and difficulty in retrieving captured CTCs from the devices for further laboratory analysis. Moreover, the white blood cells that contaminate trapped CTCs, which are similar in size, can be mistakenly regarded as CTCs; this is a limitation associated with these devices.
Increasing global prevalence of cancer is one of the major drivers for growth of the CTC market. In 2023, the Pan American Health Organization reported around 20 million newly diagnosed cancer cases and 10 million cancer-related deaths. Projections indicate a probable 60% surge in cancer burden over the next two decades, posing additional challenges to healthcare systems, individuals, and communities. It is anticipated that the global incidence of new cancer cases expected rise to approximately 30 million by the year 2040.
In addition, circulating tumor cells in breast cancer diagnosis are released in the bloodstream by cancerous tumors. As per the Breast Cancer Statistics and Resources, it is anticipated that in 2023, around 297,790 women might be diagnosed of breast cancer in the U.S., solidifying its status as the most prevalent cancer among American women. The rising burden of cancer also attracts increased investment in research and development for new cancer therapies & technologies. CTCs that actively participate in clinical trials and adopt innovative treatment options can attract patients seeking access to cutting-edge care, further boosting their market position.
Since the last several decades, extensive translational and clinical cancer research programs in the field of CTCs are being conducted. The ongoing research on circulating tumor cells technology by several government bodies, such as the American Association of Cancer Research and the American Society of Clinical Oncology, to utilize it as a surrogate marker for the determination of cancer progression is expected to aid the growth of the market.
Furthermore, rising adoption of biomarker tests enables minimally invasive screening of tumors before opting for complex surgical procedures, such as radiotherapy, chemotherapy, and surgical removal of tumors. Therefore, the high demand for noninvasive procedures due to their simpler nature is anticipated to have a considerable impact on the CTC market over the forecast period.
Market Concentration & Characteristics
The circulating tumor cells market has seen significant innovation in recent years. Some of the recent innovations include the development of microfluidic chips for CTC capture, the use of imaging techniques for circulating tumor cells detection, and the implementation of machine learning algorithms for CTC analysis. These advances have the potential to revolutionize cancer care by providing more accurate and reliable information about a patient's disease status.
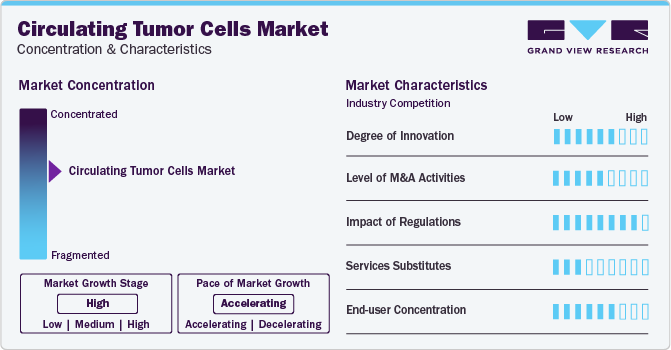
The CTC market is also characterized by a high level of merger and acquisition (M&A) activity by the leading players. M&A drives companies to focus on specific applications of CTC technology, such as early cancer detection, treatment monitoring, or personalized medicine. This could lead to the development of more targeted and effective circulating tumor cells tests and therapies.
Regulation can have a significant impact on the CTC market. Regulatory bodies such as the FDA play a crucial role in determining the safety and efficacy of CTC products and services. Stringent regulations can lead to delays in the approval of new CTC products, which can slow down innovation and limit the options available to patients and healthcare providers.
In this market, substitute services refer to alternative products or solutions that can replace or supplement the use of CTC technologies. Some examples of substitute services in the CTC market include traditional diagnostic methods such as biopsies, blood tests, and imaging technologies. While CTC technologies offer many benefits, including noninvasive testing and early detection of cancer, substitute services still be necessary in certain situations.
End-user concentration in the CTC market is relatively high, with the majority of tests being performed in hospitals, diagnostic laboratories, and research institutions. However, there is also a growing trend towards point-of-care testing, which allows for faster and more convenient testing in a variety of settings, including clinics and physician offices.
Technology Insights
In 2023, the CTC detection and enrichment methods segment accounted for the largest revenue share of 66.21%. The availability of different methods for the enrichment of circulating tumor cells in cancer detection is expected to significantly impact segment growth over the forecast period. Moreover, positive or negative enrichment of CTCs based on biological properties is expected to hold significant potential for market growth. An effective enrichment process helps in the enhancement of sensitivity, selectivity, and yield, thereby ensuring successful clinical translation of this field. Different techniques for CTC detection include magnetic beads-based centrifugal force, enrichment, filtration, and other physical properties such as density, deformity, size, & electric charges.
The CTC analysis segment is expected to register the fastest CAGR from 2024 to 2030. Understanding subclonal intratumor heterogeneity using CTC detection can help clinicians in determining the underlying reasons for unresponsive patients subjected to targeted therapy, eventually leading to enhanced cancer therapies. Hence, CTC analysis is a minimally invasive method in repetitive analysis and validated technology for tumor study & clinical decision making. Technological advancements such as immunofluorescence, Next-Generation Sequencing (NGS), Fluorescence In Situ Hybridization (FISH), and quantitative PCR (qPCR) are anticipated to improve CTC analyses. Hence, due to the presence of fundamental principle of amplification in qPCR, the detection of CTCs in a large blood sample becomes efficient and accurate.
Application Insights
The research segment dominated the market and captured the largest revenue share in 2023. CTCs are regarded as a substrate of cancer metastasis. Currently, the enumeration of CTCs represents an effective predictive and prognostic biomarker capable of monitoring the efficacy of adjuvant therapies, identifying early development of metastases, and evaluating therapeutic responses better than conventional imaging methods. In addition, CTC enumeration remains largely a research tool.
Recently, the focus has shifted toward CTC characterization and isolation, which can provide significant opportunities in predictive testing research. For instance, in April 2021, Bio-Techne Corporation acquired Asuragen, Inc. for USD 215 million. Asuragen’s expertise in productizing lab-developed tests and commercializing innovative molecular products allowed Bio-Techne to offer advanced solutions to researchers and clinicians.
Clinical/ liquid biopsy is expected to register a significant CAGR from 2024 to 2030. In a liquid biopsy, the analysis CTCs in a cancer patient’s bloodstream has received significant attention for their potential clinical utility. The number of CTCs in a patient’s blood can differ based on cancer type, stage, and treatment. The number of CTCs found in the patient’s bloodstream indicates the state of the disease. Patients in the later stages of cancer show a higher number of CTCs than patients in early stages of cancer.
Product Insights
The kits & reagents segment dominated the market and accounted for the largest revenue share of 2023. This can be attributed to frequent purchases and high usage rates. For instance, CellSearch Circulating Tumour Cell Kit authorized by the U.S. FDA, is one the most popular products produced in the U.S. Additionally, the availability of a robust product portfolio coupled with advancements in microfluidics technology is expected to propel the market growth.
The devices or systems segment is expected to showcase significant CAGR during the forecast period. The introduction of fabricated glass microchips to overcome the challenges and to increase technical completeness for mass production is expected to propel the segment growth. The development of automated instruments that eliminate the use of additional blood collection tubes reduces the cost of blood collection tubes.
The reduction in costs is also observed as the transportation is free of the additional laboratory consumables and transfer tubes when the reagent-equipped tubes are used. On the other hand, kits and reagents have also contributed significantly to the segment’s revenue.
End-use Insights
The research and academic institutes segment accounted for the largest revenue share of 2023. This can be attributed to the increasing focus on research and development activities for cancer diagnosis and treatment. These institutes are investing heavily in advanced technologies and equipment to enhance the accuracy and efficiency of circulating tumor cell detection and analysis.
The diagnostic centers segment is expected to register a significant CAGR from 2024 to 2030. This is due to their advanced technology and expertise in detecting and analyzing CTCs. They have access to advanced equipment and trained professionals who can perform accurate and reliable tests. Additionally, diagnostic centers have partnerships with hospitals and healthcare providers, which gives them a larger customer base and more opportunities to expand their services. These factors have contributed to their success in the CTC market.
Specimen Insights
The blood specimen segment dominated the market and accounted for the largest revenue share in 2023. A large concentration of these cells in blood samples as compared with other biospecimens is responsible for the largest penetration of this specimen type. Approaches for tumor cell identification in blood samples are considered important in current cancer research, as it aids in the prediction of prognosis and determination of the response to systemic chemotherapy. However, the use of whole blood as a specimen poses a challenge when combined with microfluidic technology.
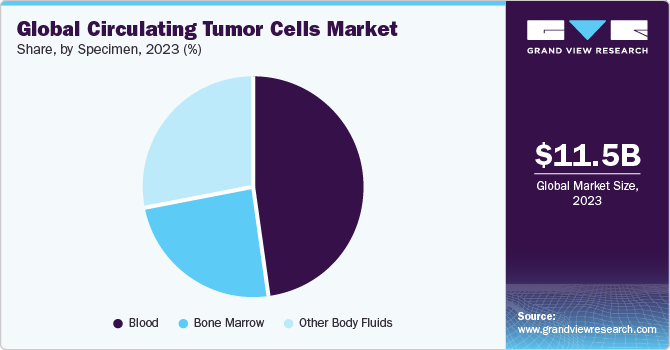
The bone marrow segment is expected to register a significant CAGR during the forecast period. Bone marrow samples, when analyzed for CTCs, play a vital role in cancer research and clinical trials. Researchers use these samples to study the biology of circulating tumor cells, understand the mechanisms of metastasis, and develop novel therapeutic approaches. These factors are expected to support segment growth in the forecast years.
Regional Insights
North America captured the largest revenue share of 42.33% in 2023. Aviva Biosciences; Apocell, Inc.; Advanced Cell Diagnostics; Biocept, Inc.; Biofluidica, Inc.; and CellTraffix, Inc. are the key players operating in the region. These players are undertaking various strategies to enhance their market hold, which is a key factor responsible for the high share of the U.S. market for circulating tumor cells.
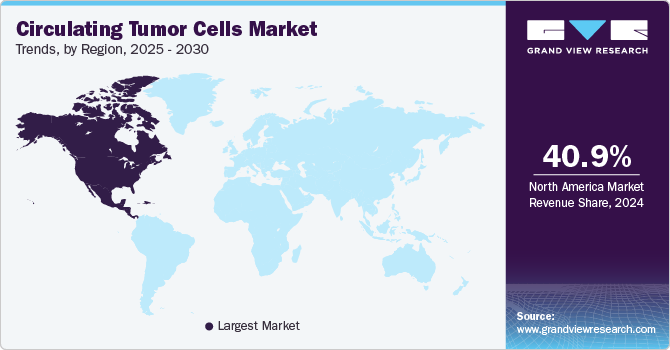
The U.S. is anticipated to significantly contribute to the regional market's growth due to factors including the rising incidence of cancer, product approvals, and increased R&D efforts. For instance, the American Cancer Society projects that there approximately 127,070 lung cancer fatalities nationwide and about 238,340 new instances of lung cancer diagnosis in 2023. Furthermore, the presence of a population with high susceptibility to cancer, an increase in market penetration rates, and technologically advanced cancer care infrastructure are supporting the region’s growth.
Asia Pacific is projected to grow at a lucrative CAGR during the forecast period, due to high unmet diagnostic needs coupled with rapidly growing awareness with regard to early detection of cancer and risk assessment. China is a major country that drives the regional market growth. The economy of China developed its infrastructure with academic and research institutes that provide knowledge and create interest in the emerging fields of diagnostics, biotechnology, & genetics. It also created facilities to support their development by adopting the latest technologies. The competitive nature of the government, leading to high investments in emerging fields of research, is anticipated to boost revenue generation over the forecast period.
Key Circulating Tumor Cells Company Insights
Partnerships, acquisitions, and expansions are some of the most adopted strategies by the key players. Companies such as Bio-Techne have acquired smaller companies with advanced technologies to expand their market reach globally.
Key Circulating Tumor Cells Companies:
The following are the leading companies in the circulating tumor cells market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these CTC companies are analyzed to map the supply network.
- QIAGEN
- Bio-Techne Corporation
- Precision for Medicine
- AVIVA Biosciences
- BIOCEPT, Inc.
- BioCEP Ltd.
- Fluxion Biosciences, Inc.
- Greiner Bio-One International GmbH
- Ikonisys, Inc.
- Miltenyi Biotec
- IVDiagnostics, Inc.
- BioFluidica
- Canopus Bioscience Ltd.
- Biolidics Limited
- Creativ MicroTech, Inc.
- LungLife AI, Inc.
- Epic Sciences
- Rarecells Diagnostics
- ScreenCell
- Menarini Silicon Biosystems
- LineaRx, Inc. (Vitatex, Inc.)
- Sysmex Corporation
- STEMCELL Technologies, Inc.
Recent Developments
-
In August 2023, Cell Microsystems acquired Fluxion Biosciences, Inc. This acquisition enabled precise measurement of electrical currents across cell membranes, providing insights into ion channel activity and cell signaling.
-
In June 2023, Bio-Techne announced the acquisition of LunaPhore, a Swiss company developing advanced technologies for tissue imaging and analysis. By integrating LunaPhore's expertise into its existing portfolio, Bio-Techne aimed to create a comprehensive, end-to-end workflow for researchers studying tissues. The acquisition is expected to close in the first quarter of 2024.
-
In May 2023, Menarini Silicon Biosystems partnered with Alivio Health to provide access to its CELLSEARCH liquid biopsy tests for Alivio Health's clients and members. CELLSEARCH tests provide noninvasive analysis of CTCs to help in clinical decision-making, potentially leading to earlier diagnosis and more effective treatment strategies.
-
In March 2023, Miltenyi Biotec acquired Lino Biotech AG. This acquisition of Lino Biotech's innovative biosensor technology was expected to help in the development of new & improved assays and quality control processes in the field.
Circulating Tumor Cells Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 12.81 billion
Revenue forecast in 2030
USD 27.55 billion
Growth rate
CAGR of 13.62% from 2024 to 2030
Actual Data
2018 - 2023
Forecast period
2024 - 2030
Report updated
January 2024
Quantitative units
Revenue in USD billion/million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Technology, application, product, specimen, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Sweden; Denmark; Norway; China; Japan; India; South Korea; Australia; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; Kuwait; UAE; South Africa
Key companies profiled
QIAGEN; Bio-Techne Corp.; Precision for Medicine; AVIVA Biosciences; BIOCEPT, Inc.; BioCEP Ltd.; Fluxion Biosciences, Inc.; Greiner Bio-One International GmbH; Ikonisys, Inc.; Miltenyi Biotec; IVDiagnostics; BioFluidica; Canopus Bioscience Ltd.; Biolidics Limited; Creativ MicroTech, Inc.; LungLife AI, Inc.; Epic Sciences; Rarecells Diagnostics; ScreenCell; Menarini Silicon Biosystems; LineaRx, Inc. (Vitatex, Inc.); Sysmex Corporation; STEMCELL Technologies, Inc.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Circulating Tumor Cells Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global circulating tumor cells market report based on technology, application, product, specimen, end-use, and region:
-
Technology Outlook (Revenue, USD Billion, 2018 - 2030)
-
CTC Detection & Enrichment Methods
-
Immunocapture (Label-based)
-
Positive Selection
-
Negative Selection
-
-
Size-based Separation (Label-free)
-
Membrane-based
-
Microfluidic-based
-
-
Density-based Separation (Label-free)
-
Combined Methods
-
-
CTC Direct Detection Methods
-
SERS
-
Microscopy
-
Others
-
-
CTC Analysis
-
-
Application Outlook (Revenue, USD Billion, 2018 - 2030)
-
Clinical/ Liquid Biopsy
-
Risk Assessment
-
Screening and Monitoring
-
-
Research
-
Cancer Stem Cell & Tumorogenesis Research
-
Drug/Therapy Development
-
-
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Kits & Reagents
-
Blood Collection Tubes
-
Devices or Systems
-
-
Specimen Outlook (Revenue, USD Billion, 2018 - 2030)
-
Blood
-
Bone Marrow
-
Other Body Fluids
-
-
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Research and Academic Institutes
-
Hospital and Clinics
-
Diagnostic Centers
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Sweden
-
Denmark
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
UAE
-
Saudi Arabia
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global circulating tumor cells market size was estimated at USD 11.47 billion in 2023 and is expected to reach USD 27.55 billion in 2023.
b. The global circulating tumor cells market is expected to grow at a compound annual growth rate of 13.62% from 2024 to 2030 to reach USD 27.55 billion by 2030.
b. CTC detection & enrichment methods dominated the CTC market with a share of 66.21% in 2023. This is attributable to the availability of different methods for the enrichment of circulating tumor cells in cancer detection.
b. Some key players operating in the CTCs market include QIAGEN, Bio-Techne Corporation, BIOCEPT, Inc., Greiner Bio One International GmbH, Miltenyi Biotec, Biolidics Limited, Creatv MicroTech, Inc. and among others.
b. Key factors that are driving the circulating tumor cells market growth include rising cancer prevalence, increasing preference for non-invasive cancer diagnosis, and technological advancements in CTC isolation and analysis.
Table of Contents
Chapter 1. Circulating Tumor Cells Market: Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.2.1. Technology
1.2.2. Application
1.2.3. Product
1.2.4. Specimen
1.2.5. End-use
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. GVR’s internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.4.5. Details of primary research
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Approach 1: Commodity flow approach
1.7.3. Volume price analysis (Model 2)
1.7.4. Approach 2: Volume price analysis
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
Chapter 2. Circulating Tumor Cells Market: Executive Summary
2.1. Market Outlook
2.2. Segment Snapshot
2.3. Competitive Insights
Chapter 3. Circulating Tumor Cells Market: Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. Advancements in chip technology
3.2.1.2. Expanding applications of CTCs
3.2.1.3. Growing demand for early and rapid cancer diagnosis
3.2.1.4. Growing incidence of cancer
3.2.2. Market restraint analysis
3.2.2.1. Consistency-related challenges in CTC detection and enrichment
3.2.2.2. Higher preference for Point-of-Care (POC) testing and nonavailability of POC adaptable CTC tests
3.2.2.3. Lower applicability of CTCs in rare cancers
3.3. Circulating Tumor Cells Market Analysis Tools
3.3.1. Industry Analysis - Porter’s
3.3.2. PESTEL Analysis
3.3.3. COVID-19 Impact Analysis
Chapter 4. Circulating Tumor Cells Market: Technology Estimates & Trend Analysis
4.1. Technology Market Share, 2023 & 2030
4.2. Global Circulating Tumor Cells Market by Technology Outlook
4.3. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
4.3.1. CTC Detection & Enrichment Methods
4.3.1.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.2. Immunocapture (Label-based)
4.3.1.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.2.2. Positive Selection
4.3.1.2.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.2.3. Negative Selection
4.3.1.2.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.3. Size-based Separation (Label-free)
4.3.1.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.3.2. Membrane-based
4.3.1.3.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.3.3. Microfluidic-based
4.3.1.3.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.4. Density-based Separation (Label-free)
4.3.1.4.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.1.5. Combined Methods
4.3.1.5.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.2. CTC Direct Detection Methods
4.3.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.2.2. SERS
4.3.2.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.2.3. Microscopy
4.3.2.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.2.4. Others
4.3.2.4.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.3. CTC Analysis
4.3.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 5. Circulating Tumor Cells Market: Application Estimates & Trend Analysis
5.1. Application Market Share, 2023 & 2030
5.2. Global Circulating Tumor Cells Market by Application Outlook
5.3. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
5.3.1. Clinical/ Liquid Biopsy
5.3.1.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
5.3.1.2. Risk Assessment
5.3.1.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
5.3.1.3. Screening and Monitoring
5.3.1.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
5.3.2. Research
5.3.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
5.3.2.2. Cancer Stem Cell & Tumorogenesis Research
5.3.2.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
5.3.2.3. Drug/Therapy Development
5.3.2.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 6. Circulating Tumor Cells Market: Product Estimates & Trend Analysis
6.1. Product Market Share, 2023 & 2030
6.2. Global Circulating Tumor Cells Market by Product Outlook
6.3. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
6.3.1. Kits & reagents
6.3.1.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
6.3.2. Blood collection tubes
6.3.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
6.3.3. Devices or systems
6.3.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 7. Circulating Tumor Cells Market: Specimen Estimates & Trend Analysis
7.1. Specimen Market Share, 2023 & 2030
7.2. Global Circulating Tumor Cells Market by Specimen Outlook
7.3. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
7.3.1. Blood
7.3.1.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.2. Bone marrow
7.3.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.3. Other body fluids
7.3.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 8. Circulating Tumor Cells Market: End-use Estimates & Trend Analysis
8.1. End-use Market Share, 2023 & 2030
8.2. Global Circulating Tumor Cells Market by End-use Outlook
8.3. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
8.3.1. Research and academic institutes
8.3.1.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
8.3.2. Hospital and clinics
8.3.2.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
8.3.3. Diagnostic centers
8.3.3.1. Market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 9. Circulating Tumor Cells Market: Regional Estimates & Trend Analysis
9.1. Regional Market Share Analysis, 2023 & 2030
9.2. North America
9.2.1. North America market estimates and forecasts, 2018 - 2030 (USD Million)
9.2.2. U.S.
9.2.2.1. Key country dynamics
9.2.2.2. Regulatory framework
9.2.2.3. Competitive scenario
9.2.2.4. U.S. market estimates and forecasts, 2018 - 2030 (USD Million)
9.2.2.5. Target disease prevalence
9.2.3. Canada
9.2.3.1. Key country dynamics
9.2.3.2. Regulatory framework
9.2.3.3. Competitive scenario
9.2.3.4. Canada market estimates and forecasts, 2018 - 2030 (USD Million)
9.2.3.5. Target disease prevalence
9.3. Europe
9.3.1. Europe market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.2. UK
9.3.2.1. Key country dynamics
9.3.2.2. Regulatory framework
9.3.2.3. Competitive scenario
9.3.2.4. UK market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.2.5. Target disease prevalence
9.3.3. Germany
9.3.3.1. Key country dynamics
9.3.3.2. Regulatory framework
9.3.3.3. Competitive scenario
9.3.3.4. Germany market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.3.5. Target disease prevalence
9.3.4. France
9.3.4.1. Key country dynamics
9.3.4.2. Regulatory framework
9.3.4.3. Competitive scenario
9.3.4.4. France market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.4.5. Target disease prevalence
9.3.5. Italy
9.3.5.1. Key country dynamics
9.3.5.2. Regulatory framework
9.3.5.3. Competitive scenario
9.3.5.4. Italy market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.5.5. Target disease prevalence
9.3.6. Spain
9.3.6.1. Key country dynamics
9.3.6.2. Regulatory framework
9.3.6.3. Competitive scenario
9.3.6.4. Spain market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.6.5. Target disease prevalence
9.3.7. Norway
9.3.7.1. Key country dynamics
9.3.7.2. Regulatory framework
9.3.7.3. Competitive scenario
9.3.7.4. Norway market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.7.5. Target disease prevalence
9.3.8. Sweden
9.3.8.1. Key country dynamics
9.3.8.2. Regulatory framework
9.3.8.3. Competitive scenario
9.3.8.4. Sweden market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.8.5. Target disease prevalence
9.3.9. Denmark
9.3.9.1. Key country dynamics
9.3.9.2. Regulatory framework
9.3.9.3. Competitive scenario
9.3.9.4. Denmark market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.9.5. Target disease prevalence
9.4. Asia Pacific
9.4.1. Asia Pacific market estimates and forecasts, 2018 - 2030 (USD Million)
9.4.2. Japan
9.4.2.1. Key country dynamics
9.4.2.2. Regulatory framework
9.4.2.3. Competitive scenario
9.4.2.4. Japan market estimates and forecasts, 2018 - 2030 (USD Million)
9.4.2.5. Target disease prevalence
9.4.3. China
9.4.3.1. Key country dynamics
9.4.3.2. Regulatory framework
9.4.3.3. Competitive scenario
9.4.3.4. China market estimates and forecasts, 2018 - 2030 (USD Million)
9.4.3.5. Target disease prevalence
9.4.4. India
9.4.4.1. Key country dynamics
9.4.4.2. Regulatory framework
9.4.4.3. Competitive scenario
9.4.4.4. India market estimates and forecasts, 2018 - 2030 (USD Million)
9.4.4.5. Target disease prevalence
9.4.5. Australia
9.4.5.1. Key country dynamics
9.4.5.2. Regulatory framework
9.4.5.3. Competitive scenario
9.4.5.4. Australia market estimates and forecasts, 2018 - 2030 (USD Million)
9.4.5.5. Target disease prevalence
9.4.6. South Korea
9.4.6.1. Key country dynamics
9.4.6.2. Regulatory framework
9.4.6.3. Competitive scenario
9.4.6.4. South Korea market estimates and forecasts, 2018 - 2030 (USD Million)
9.4.6.5. Target disease prevalence
9.4.7. Thailand
9.4.7.1. Key country dynamics
9.4.7.2. Regulatory framework
9.4.7.3. Competitive scenario
9.4.7.4. Thailand market estimates and forecasts, 2018 - 2030 (USD Million)
9.4.7.5. Target disease prevalence
9.5. Latin America
9.5.1. Latin America market estimates and forecasts, 2018 - 2030 (USD Million)
9.5.2. Brazil
9.5.2.1. Key country dynamics
9.5.2.2. Regulatory framework
9.5.2.3. Competitive scenario
9.5.2.4. Brazil market estimates and forecasts, 2018 - 2030 (USD Million)
9.5.2.5. Target disease prevalence
9.5.3. Mexico
9.5.3.1. Key country dynamics
9.5.3.2. Regulatory framework
9.5.3.3. Competitive scenario
9.5.3.4. Mexico market estimates and forecasts, 2018 - 2030 (USD Million)
9.5.3.5. Target disease prevalence
9.5.4. Argentina
9.5.4.1. Key country dynamics
9.5.4.2. Regulatory framework
9.5.4.3. Competitive scenario
9.5.4.4. Argentina market estimates and forecasts, 2018 - 2030 (USD Million)
9.5.4.5. Target disease prevalence
9.6. MEA
9.6.1. MEA market estimates and forecasts, 2018 - 2030 (USD Million)
9.6.2. South Africa
9.6.2.1. Key country dynamics
9.6.2.2. Regulatory framework
9.6.2.3. Competitive scenario
9.6.2.4. South Africa market estimates and forecasts, 2018 - 2030 (USD Million)
9.6.2.5. Target disease prevalence
9.6.3. Saudi Arabia
9.6.3.1. Key country dynamics
9.6.3.2. Regulatory framework
9.6.3.3. Competitive scenario
9.6.3.4. Saudi Arabia market estimates and forecasts, 2018 - 2030 (USD Million)
9.6.3.5. Target disease prevalence
9.6.4. UAE
9.6.4.1. Key country dynamics
9.6.4.2. Regulatory framework
9.6.4.3. Competitive scenario
9.6.4.4. UAE market estimates and forecasts, 2018 - 2030 (USD Million)
9.6.4.5. Target disease prevalence
9.6.5. Kuwait
9.6.5.1. Key country dynamics
9.6.5.2. Regulatory framework
9.6.5.3. Competitive scenario
9.6.5.4. Kuwait market estimates and forecasts, 2018 - 2030 (USD Million)
9.6.5.5. Target disease prevalence
Chapter 10. Competitive Landscape
10.1. Recent Developments & Impact Analysis, By Key Market Participants
10.2. Company/Competition Categorization
10.3. Vendor Landscape
10.3.1. List of key distributors and channel partners
10.3.2. Key customers
10.3.3. Key company position analysis, 2023
10.4. Company Profiles
10.4.1. QIAGEN
10.4.1.1. Company overview
10.4.1.2. Financial performance
10.4.1.3. Product benchmarking
10.4.1.4. Strategic initiatives
10.4.2. Bio-Techne Corp.
10.4.2.1. Company overview
10.4.2.2. Financial performance
10.4.2.3. Product benchmarking
10.4.2.4. Strategic initiatives
10.4.3. Precision for Medicine
10.4.3.1. Company overview
10.4.3.2. Financial performance
10.4.3.3. Product benchmarking
10.4.3.4. Strategic initiatives
10.4.4. AVIVA Biosciences
10.4.4.1. Company overview
10.4.4.2. Financial performance
10.4.4.3. Product benchmarking
10.4.4.4. Strategic initiatives
10.4.5. BIOCEPT, Inc.
10.4.5.1. Company overview
10.4.5.2. Financial performance
10.4.5.3. Product benchmarking
10.4.5.4. Strategic initiatives
10.4.6. BioCEP Ltd.
10.4.6.1. Company overview
10.4.6.2. Financial performance
10.4.6.3. Product benchmarking
10.4.6.4. Strategic initiatives
10.4.7. Fluxion Biosciences, Inc.
10.4.7.1. Company overview
10.4.7.2. Financial performance
10.4.7.3. Product benchmarking
10.4.7.4. Strategic initiatives
10.4.8. Greiner Bio-One International GmbH
10.4.8.1. Company overview
10.4.8.2. Financial performance
10.4.8.3. Product benchmarking
10.4.8.4. Strategic initiatives
10.4.9. Ikonisys, Inc.
10.4.9.1. Company overview
10.4.9.2. Financial performance
10.4.9.3. Product benchmarking
10.4.9.4. Strategic initiatives
10.4.10. Miltenyi Biotec
10.4.10.1. Company overview
10.4.10.2. Financial performance
10.4.10.3. Product benchmarking
10.4.10.4. Strategic initiatives
10.4.11. IVDiagnostics
10.4.11.1. Company overview
10.4.11.2. Financial performance
10.4.11.3. Product benchmarking
10.4.11.4. Strategic initiatives
10.4.12. BioFluidica
10.4.12.1. Company overview
10.4.12.2. Financial performance
10.4.12.3. Product benchmarking
10.4.12.4. Strategic initiatives
10.4.13. Canopus Bioscience Ltd.
10.4.13.1. Company overview
10.4.13.2. Financial performance
10.4.13.3. Product benchmarking
10.4.13.4. Strategic initiatives
10.4.14. Biolidics Limited
10.4.14.1. Company overview
10.4.14.2. Financial performance
10.4.14.3. Product benchmarking
10.4.14.4. Strategic initiatives
10.4.15. Creativ MicroTech , Inc.
10.4.15.1. Company overview
10.4.15.2. Financial performance
10.4.15.3. Product benchmarking
10.4.15.4. Strategic initiatives
10.4.16. LungLife AI
10.4.16.1. Company overview
10.4.16.2. Financial performance
10.4.16.3. Product benchmarking
10.4.16.4. Strategic initiatives
10.4.17. Epic Sciences
10.4.17.1. Company overview
10.4.17.2. Financial performance
10.4.17.3. Product benchmarking
10.4.17.4. Strategic initiatives
10.4.18. Rarecells Diagnostics
10.4.18.1. Company overview
10.4.18.2. Financial performance
10.4.18.3. Product benchmarking
10.4.18.4. Strategic initiatives
10.4.19. ScreenCell
10.4.19.1. Company overview
10.4.19.2. Financial performance
10.4.19.3. Product benchmarking
10.4.19.4. Strategic initiatives
10.4.20. Menarini Silicon Biosystems
10.4.20.1. Company overview
10.4.20.2. Financial performance
10.4.20.3. Product benchmarking
10.4.20.4. Strategic initiatives
10.4.21. LineaRx, Inc. (Vitatex, Inc.)
10.4.21.1. Company overview
10.4.21.2. Financial performance
10.4.21.3. Product benchmarking
10.4.21.4. Strategic initiatives
10.4.22. Sysmex Corporation
10.4.22.1. Company overview
10.4.22.2. Financial performance
10.4.22.3. Product benchmarking
10.4.22.4. Strategic initiatives
10.4.23. STEMCELL Technologies, Inc.
10.4.23.1. Company overview
10.4.23.2. Financial performance
10.4.23.3. Product benchmarking
10.4.23.4. Strategic initiatives
List of Tables
Table 1 List of abbreviation
Table 2 North America circulating tumor cells market, by region, 2018 - 2030 (USD Million)
Table 3 North America circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 4 North America circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 5 North America circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 6 North America circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 7 North America circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 8 U.S. circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 9 U.S. circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 10 U.S. circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 11 U.S. circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 12 U.S. circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 13 Canada circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 14 Canada circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 15 Canada circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 16 Canada circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 17 Canada circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 18 Europe circulating tumor cells market, by region, 2018 - 2030 (USD Million)
Table 19 Europe circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 20 Europe circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 21 Europe circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 22 Europe circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 23 Europe circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 24 UK circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 25 UK circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 26 UK circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 27 UK circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 28 UK circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 29 Germany circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 30 Germany circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 31 Germany circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 32 Germany circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 33 Germany circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 34 Italy circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 35 Italy circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 36 Italy circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 37 Italy circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 38 Italy circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 39 France circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 40 France circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 41 France circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 42 France circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 43 France circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 44 Spain circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 45 Spain circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 46 Spain circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 47 Spain circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 48 Spain circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 49 Sweden circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 50 Sweden circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 51 Sweden circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 52 Sweden circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 53 Sweden circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 54 Denmark circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 55 Denmark circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 56 Denmark circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 57 Denmark circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 58 Denmark circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 59 Norway circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 60 Norway circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 61 Norway circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 62 Norway circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 63 Norway circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 64 Asia Pacific circulating tumor cells market, by region, 2018 - 2030 (USD Million)
Table 65 Asia Pacific circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 66 Asia Pacific circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 67 Asia Pacific circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 68 Asia Pacific circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 69 Asia Pacific circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 70 China circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 71 China circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 72 China circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 73 China circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 74 China circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 75 Japan circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 76 Japan circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 77 Japan circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 78 Japan circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 79 Japan circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 80 India circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 81 India circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 82 India circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 83 India circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 84 India circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 85 South Korea circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 86 South Korea circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 87 South Korea circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 88 South Korea circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 89 South Korea circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 90 Australia circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 91 Australia circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 92 Australia circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 93 Australia circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 94 Australia circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 95 Thailand circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 96 Thailand circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 97 Thailand circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 98 Thailand circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 99 Thailand circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 100 Latin America circulating tumor cells market, by region, 2018 - 2030 (USD Million)
Table 101 Latin America circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 102 Latin America circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 103 Latin America circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 104 Latin America circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 105 Latin America circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 106 Brazil circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 107 Brazil circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 108 Brazill circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 109 Brazil circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 110 Brazil circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 111 Mexico circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 112 Mexico circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 113 Mexico circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 114 Mexico circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 115 Mexico circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 116 Argentina circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 117 Argentina circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 118 Argentina circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 119 Argentina circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 120 Argentina circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 121 Middle East & Africa circulating tumor cells market, by region, 2018 - 2030 (USD Million)
Table 122 Middle East & Africa circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 123 Middle East & Africa circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 124 Middle East & Africa circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 125 Middle East & Africa circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 126 Middle East & Africa circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 127 South Africa circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 128 South Africa circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 129 South Africa circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 130 South Africa circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 131 South Africa circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 132 Saudi Arabia circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 133 Saudi Arabia circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 134 Saudi Arabia circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 135 Saudi Arabia circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 136 Saudi Arabia circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 137 UAE circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 138 UAE circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 139 UAE circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 140 UAE circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 141 UAE circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
Table 142 Kuwait circulating tumor cells market, by technology, 2018 - 2030 (USD Million)
Table 143 Kuwait circulating tumor cells market, by application, 2018 - 2030 (USD Million)
Table 144 Kuwait circulating tumor cells market, by product, 2018 - 2030 (USD Million)
Table 145 Kuwait circulating tumor cells market, by specimen, 2018 - 2030 (USD Million)
Table 146 Kuwait circulating tumor cells market, by end-use, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Market research process
Fig. 2 Data triangulation techniques
Fig. 3 Primary research pattern
Fig. 4 Market research approaches
Fig. 5 Value-chain-based sizing & forecasting
Fig. 6 QFD modeling for market share assessment
Fig. 7 Market formulation & validation
Fig. 8 Circulating tumor cells market: market outlook
Fig. 9 Circulating tumor cells competitive insights
Fig. 10 Parent market outlook
Fig. 11 Related/ancillary market outlook
Fig. 12 Penetration and growth prospect mapping
Fig. 13 Industry value chain analysis
Fig. 14 Circulating tumor cells market driver impact
Fig. 15 Circulating tumor cells market restraint impact
Fig. 16 Circulating tumor cells market strategic initiatives analysis
Fig. 17 Circulating tumor cells market: Technology movement analysis
Fig. 18 Circulating tumor cells market: Technology outlook and key takeaways
Fig. 19 CTC detection & enrichment methods market estimates and forecast, 2018 - 2030
Fig. 20 Immunocapture (label-based) estimates and forecast, 2018 - 2030
Fig. 21 Positive selection market estimates and forecast, 2018 - 2030
Fig. 22 Negative selection estimates and forecast, 2018 - 2030
Fig. 23 Size-based separation (label-free) market estimates and forecast, 2018 - 2030
Fig. 24 Membrane-based estimates and forecast, 2018 - 2030
Fig. 25 Microfluidic-based estimates and forecast, 2018 - 2030
Fig. 26 Density-based Separation (Label-free) estimates and forecast, 2018 - 2030
Fig. 27 Combined Methods estimates and forecast, 2018 - 2030
Fig. 28 CTC direct detection methods estimates and forecast, 2018 - 2030
Fig. 29 SERS estimates and forecast, 2018 - 2030
Fig. 30 Microscopy estimates and forecast, 2018 - 2030
Fig. 31 Others estimates and forecast, 2018 - 2030
Fig. 32 CTC Analysis estimates and forecast, 2018 - 2030
Fig. 33 Circulating tumor cells Market: Application movement Analysis
Fig. 34 Circulating tumor cells market: Application outlook and key takeaways
Fig. 35 Clinical/ Liquid Biopsy market estimates and forecasts, 2018 - 2030
Fig. 36 Risk Assessment market estimates and forecasts, 2018 - 2030
Fig. 37 Screening and Monitoring market estimates and forecasts, 2018 - 2030
Fig. 38 Research market estimates and forecasts, 2018 - 2030
Fig. 39 Cancer Stem Cell & Tumorogenesis Research market estimates and forecasts, 2018 - 2030
Fig. 40 Drug/Therapy Development market estimates and forecasts, 2018 - 2030
Fig. 41 Circulating tumor cells Market: Product movement Analysis
Fig. 42 Circulating tumor cells market: Product outlook and key takeaways
Fig. 43 Kits & reagents market estimates and forecasts, 2018 - 2030
Fig. 44 Blood collection tubes market estimates and forecasts, 2018 - 2030
Fig. 45 Devices or Systems market estimates and forecasts, 2018 - 2030
Fig. 46 Circulating tumor cells Market: Specimen movement Analysis
Fig. 47 Circulating tumor cells market: Specimen outlook and key takeaways
Fig. 48 Blood market estimates and forecasts, 2018 - 2030
Fig. 49 Bone marrow market estimates and forecasts, 2018 - 2030
Fig. 50 Other body fluids market estimates and forecasts, 2018 - 2030
Fig. 51 Circulating tumor cells Market: End-use movement Analysis
Fig. 52 Circulating tumor cells market: End-use outlook and key takeaways
Fig. 53 Research and academic institutes market estimates and forecasts, 2018 - 2030
Fig. 54 Hospital and clinics market estimates and forecasts, 2018 - 2030
Fig. 55 Diagnostic centers market estimates and forecasts, 2018 - 2030
Fig. 56 Global circulating tumor cells market: Regional movement analysis
Fig. 57 Global circulating tumor cells market: Regional outlook and key takeaways
Fig. 58 North America, by country
Fig. 59 North America
Fig. 60 North America market estimates and forecasts, 2018 - 2030
Fig. 61 U.S.
Fig. 62 U.S. market estimates and forecasts, 2018 - 2030
Fig. 63 Canada
Fig. 64 Canada market estimates and forecasts, 2018 - 2030
Fig. 65 Europe
Fig. 66 Europe market estimates and forecasts, 2018 - 2030
Fig. 67 UK
Fig. 68 UK market estimates and forecasts, 2018 - 2030
Fig. 69 Germany
Fig. 70 Germany market estimates and forecasts, 2018 - 2030
Fig. 71 France
Fig. 72 France market estimates and forecasts, 2018 - 2030
Fig. 73 Italy
Fig. 74 Italy market estimates and forecasts, 2018 - 2030
Fig. 75 Spain
Fig. 76 Spain market estimates and forecasts, 2018 - 2030
Fig. 77 Denmark
Fig. 78 Denmark market estimates and forecasts, 2018 - 2030
Fig. 79 Sweden
Fig. 80 Sweden market estimates and forecasts, 2018 - 2030
Fig. 81 Norway
Fig. 82 Norway market estimates and forecasts, 2018 - 2030
Fig. 83 Asia Pacific
Fig. 84 Asia Pacific market estimates and forecasts, 2018 - 2030
Fig. 85 China
Fig. 86 China market estimates and forecasts, 2018 - 2030
Fig. 87 Japan
Fig. 88 Japan market estimates and forecasts, 2018 - 2030
Fig. 89 India
Fig. 90 India market estimates and forecasts, 2018 - 2030
Fig. 91 Thailand
Fig. 92 Thailand market estimates and forecasts, 2018 - 2030
Fig. 93 South Korea
Fig. 94 South Korea market estimates and forecasts, 2018 - 2030
Fig. 95 Australia
Fig. 96 Australia market estimates and forecasts, 2018 - 2030
Fig. 97 Latin America
Fig. 98 Latin America market estimates and forecasts, 2018 - 2030
Fig. 99 Brazil
Fig. 100 Brazil market estimates and forecasts, 2018 - 2030
Fig. 101 Mexico
Fig. 102 Mexico market estimates and forecasts, 2018 - 2030
Fig. 103 Argentina
Fig. 104 Argentina market estimates and forecasts, 2018 - 2030
Fig. 105 Middle East and Africa
Fig. 106 Middle East and Africa market estimates and forecasts, 2018 - 2030
Fig. 107 South Africa
Fig. 108 South Africa market estimates and forecasts, 2018 - 2030
Fig. 109 Saudi Arabia
Fig. 110 Saudi Arabia market estimates and forecasts, 2018 - 2030
Fig. 111 UAE
Fig. 112 UAE market estimates and forecasts, 2018 - 2030
Fig. 113 Kuwait
Fig. 114 Kuwait market estimates and forecasts, 2018 - 2030
Fig. 115 Market position of key market players - Circulating tumor cells marketWhat questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Circulating Tumor Cells Technology Outlook (Revenue in USD Million, 2018 - 2030)
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC Direct Detection Methods
- SERS
- Microscopy
- Others
- CTC Analysis
- CTC detection & enrichment methods
- Circulating Tumor Cells Application Outlook (Revenue in USD Million, 2018 - 2030)
- Clinical/ Liquid Biopsy
- Risk Assessment
- Screening and Monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ Liquid Biopsy
- Circulating Tumor Cells Product Outlook (Revenue in USD Million; 2018 - 2030)
- Kits & Reagents
- Blood Collection Tubes
- Devices or Systems
- Circulating Tumor Cells Specimen Outlook (Revenue in USD Million; 2018 - 2030)
- Blood
- Bone Marrow
- Other Body Fluids
- Circulating Tumor Cells End-use Outlook (Revenue in USD Million; 2018 - 2030)
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Circulating Tumor Cells Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- North America Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- North America Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- North America Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- North America Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- North America Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- U.S.
- U.S Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- U.S Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- U.S. Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- U.S Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- U.S Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- U.S Circulating Tumor Cells Market, by Technology
- Canada
- Canada Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Canada Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Canada Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Canada Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Canada Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Canada Circulating Tumor Cells Market, by Technology
- North America Circulating Tumor Cells Market, by Technology
- Europe
- Europe Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Europe Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Europe Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Europe Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Europe Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Germany
- Germany Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Germany Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Germany Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Germany Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Germany Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Germany Circulating Tumor Cells Market, by Technology
- UK
- UK Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- UK Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- UK Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- UK Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- UK Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- UK Circulating Tumor Cells Market, by Technology
- France
- France Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- France Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- France Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- France Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- France Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- France Circulating Tumor Cells Market, by Technology
- Italy
- Italy Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Italy Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Italy Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Italy Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Italy Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Italy Circulating Tumor Cells Market, by Technology
- Spain
- Spain Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Spain Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Spain Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Spain Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Spain Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Spain Circulating Tumor Cells Market, by Technology
- Sweden
- Sweden Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Sweden Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Sweden Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Sweden Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Sweden Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Sweden Circulating Tumor Cells Market, by Technology
- Denmark
- Denmark Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Denmark Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Denmark Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Denmark Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Denmark Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Denmark Circulating Tumor Cells Market, by Technology
- Norway
- Norway Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Norway Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Norway Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Norway Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Norway Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Norway Circulating Tumor Cells Market, by Technology
- Europe Circulating Tumor Cells Market, by Technology
- Asia Pacific
- Asia Pacific Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Asia Pacific Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Asia Pacific Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Asia Pacific Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Asia Pacific Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- China
- China Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- China Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- China Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- China Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- China Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- China Circulating Tumor Cells Market, by Technology
- Japan
- Japan Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Japan Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Japan Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Japan Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Japan Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Japan Circulating Tumor Cells Market, by Technology
- India
- India Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- India Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- India Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- India Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- India Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- India Circulating Tumor Cells Market, by Technology
- South Korea
- South Korea Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- South Korea Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- South Korea Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- South Korea Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- South Korea Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- South Korea Circulating Tumor Cells Market, by Technology
- Australia
- Australia Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Australia Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Australia Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Australia Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Australia Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Australia Circulating Tumor Cells Market, by Technology
- Thailand
- Thailand Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Thailand Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Thailand Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Thailand Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Thailand Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Thailand Circulating Tumor Cells Market, by Technology
- Asia Pacific Circulating Tumor Cells Market, by Technology
- Latin America
- Latin America Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Latin America Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Latin America Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Latin America Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Latin America Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Brazil
- Brazil Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Brazil Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Brazil Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Brazil Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Brazil Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Brazil Circulating Tumor Cells Market, by Technology
- Mexico
- Mexico Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Mexico Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Mexico Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Mexico Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Mexico Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Mexico Circulating Tumor Cells Market, by Technology
- Argentina
- Argentina Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Argentina Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Argentina Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Argentina Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Argentina Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Argentina Circulating Tumor Cells Market, by Technology
- Latin America Circulating Tumor Cells Market, by Technology
- Middle East and Africa (MEA)
- Middle East and Africa (MEA) Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Middle East and Africa (MEA) Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Middle East and Africa (MEA) Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Middle East and Africa (MEA) Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Middle East and Africa (MEA) Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- South Africa
- South Africa Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- South Africa Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- South Africa Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- South Africa Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- South Africa Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- South Africa Circulating Tumor Cells Market, by Technology
- UAE
- UAE Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- UAE Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- UAE Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- UAE Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- UAE Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- UAE Circulating Tumor Cells Market, by Technology
- Saudi Arabia
- Saudi Arabia Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Saudi Arabia Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Saudi Arabia Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Saudi Arabia Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Saudi Arabia Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Saudi Arabia Circulating Tumor Cells Market, by Technology
- Kuwait
- Kuwait Circulating Tumor Cells Market, by Technology
- CTC detection & enrichment methods
- Immunocapture (label-based)
- Positive selection
- Negative selection
- Size-based separation (label-free)
- Membrane-based
- Microfluidic-based
- Density-based separation (label-free)
- Combined methods
- Immunocapture (label-based)
- CTC direct detection methods
- SERS
- Microscopy
- Other
- CTC analysis
- CTC detection & enrichment methods
- Kuwait Circulating Tumor Cells Market, by Application
- Clinical/ liquid biopsy
- Risk assessment
- Screening and monitoring
- Research
- Cancer stem cell & tumorogenesis research
- Drug/therapy development
- Clinical/ liquid biopsy
- Kuwait Circulating Tumor Cells Market, by Product
- Kits & reagents
- Blood collection tubes
- Devices or systems
- Kuwait Circulating Tumor Cells Market, by Specimen
- Blood
- Bone marrow
- Other body fluids
- Kuwait Circulating Tumor Cells Market, by Specimen
- Research and academic institutes
- Hospital and clinics
- Diagnostic centers
- Kuwait Circulating Tumor Cells Market, by Technology
- Middle East and Africa (MEA) Circulating Tumor Cells Market, by Technology
- North America
Circulating Tumor Cells Market Dynamics
Drivers: Advancements In Chip Technology
The ongoing advancements in chip technology have positively impacted market growth. Cluster chip technology plays a role in capturing the exceptionally rare group of cells, such as CTCs. Isolation of CTCs is a cumbersome process and requires high accuracy. The chip technology not only helps perform precise CTC isolation but also helps overcome the challenges associated with isolation devices like lower sensitivity, high manufacturing costs, and the inability to capture CTCs of all sizes & types developed by key companies. These chips capture individual CTCs that exhibit multiple phenotypes from early- and late-stage cancer in patients. Moreover, the CTCs captured by nanotubes remain viable and can even be cultured. As these chips are transparent, it makes it easier for researchers to stain and study captured CTCs without removing them. Several researchers found that the cluster-chip technique is more efficient than a microfluidic chip, which included isolation of CTCs using antibodies that stick to the special proteins available on the surface of a tumor cell. Owing to advancements in this technology, CTC research is anticipated to witness a growth in demand for tailoring preventive medicine for cancer treatment.
Expanding Applications Of Ctcs
In recent years, CTCs are increasingly being used for determining the prognosis of cancer and helping clinicians decide the type of therapy required for cancer treatment. It can be used for multiple purposes like early detection of cancer, risk assessment of cancer recurrence, guidance for therapies, and continuous monitoring of a patient during cancer treatment. The detection of CTCs in the peripheral blood of patients with cancer acts as a promising diagnostic tool. For instance, quantitation and genomic profiling of CTCs enables cost-effective & accurate noninvasive monitoring of prostate cancer. The detection of CTCs is prognostic for the survival of cancer patients. In addition, Research organizations are constantly engaged in endeavors aimed at developing CTC-based tests, which can prove helpful in cancer diagnosis. This will positively affect the usage & adoption rates of CTCs for cancer diagnosis.
Restraints: Consistency-Related Challenges In Ctc Detection And Enrichment
CTCs can be easily detected in a patient’s bloodstream with the help of different analytical approaches, and it becomes challenging to isolate these cells & quantify their number. CTCs are a group of heterogeneous cells characterized by a diverse range of molecular and biological features. Moreover, lower number of CTCs are detected in the bloodstream as compared to other cancer biomarkers. These factors tend to impede growth to a considerable extent. Concerns related to the reliability and consistency of CTC isolation, as well as the relationship between CTC quantitation & cancer prognosis, are limiting factors affecting the clinical utility of CTCs. Although there is wide availability of several benchtop instruments for analysis and delineating CTCs from other blood cells, it is difficult to characterize CTCs due to substantial phenotypes exhibited by these cells. Considering the differences in immunophenotypical and morphologic characteristics among these cells isolated from the same tissue, CTC detection becomes more challenging, which impedes the adoption of this technique. In addition, CTCs may undergo fragmentation or get damaged, in vitro or in vivo, due to multistep cell preparation processes, thereby causing misinterpretation and subsequent inaccurate detection.
What Does This Report Include?
This section will provide insights into the contents included in this circulating tumor cells market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Circulating tumor cells market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Circulating tumor cells market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the circulating tumor cells market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for circulating tumor cells market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of circulating tumor cells market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Circulating Tumor Cells Market Categorization:
The circulating tumor cells market was categorized into six segments, namely technology (CTC Detection & Enrichment Methods, CTC Direct Detection Methods, CTC Analysis), application (Clinical/ Liquid Biopsy, Research), product (Kits & Reagents, Blood Collection Tubes, Devices or Systems), specimen (Blood, Bone Marrow), end-use (Research and Academic Institutes, Hospital and Clinics, Diagnostic Centers), and regions (North America, Europe, Asia Pacific, Latin America, Middle East & Africa).
Segment Market Methodology:
The circulating tumor cells market was segmented into technology, application, product, specimen, end-use, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The circulating tumor cells market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into twenty-five countries, namely, the U.S.; Canada; the UK; Germany; France; Italy; Spain; Benelux; Nordic Countries; Russia; China; India; Japan; South Korea; Australia; Indonesia; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; UAE; Egypt; South Africa; and Nigeria.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Circulating tumor cells market companies & financials:
The circulating tumor cells market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
QIAGEN, QIAGEN provides solutions that help customers gain molecular insights from samples containing the fundamental components of life. Its portfolio comprises a wide range of innovative products and services that cover the entire molecular testing process, from sample preparation to data analysis. The company’s offerings cater to research & development activities across various industries, including healthcare, forensics, veterinary diagnostics, food safety testing, pharmaceuticals, and biotechnology.
-
Bio-Techne Corporation, Bio-Techne Corporation is a leading developer and manufacturer of high-quality research tools and reagents used to understand & manipulate biological processes. Its product portfolio helps researchers in various fields, including life sciences research, therapeutic manufacturing, and clinical diagnostics. The company is one of the major players in the field of protein sciences, offering a comprehensive portfolio of reagents and assays for protein research. This includes antibodies, proteins, and immunoassays crucial for various applications, such as Western blotting, ELISA, & immunohistochemistry.
-
Precision Medicine Group LLC, The company supports the development of next-generation approaches for drug development and marketing. The company utilizes biomarker technology for clinical research and benefits from advancements in technology, focusing primarily on immune-response assays, genomics, biomarker analytics, specimen logistics, companion diagnostics, and clinical trial execution. The company is part of Precision Medicine Group and has 30 offices throughout the U.S., Canada, and Europe.
-
AVIVA Biosciences, The company develops technologies to advance drug discovery, life sciences, and healthcare research. The company’s products and services assist in the development of novel & safer drugs, introduce better diagnostics, and understand disease origin. The company’s recent revolutionary approach, known as RedSift Cell Processor, efficiently recovers target cells while eliminating platelets and red blood cells from the sample. The company operates as a contract research organization that provides safety screening services emphasizing on ion channels.
-
BIOCEPT, Inc., BIOCEPT, INC. is a molecular diagnostics company specializing in biomarker analysis of circulating tumor-associated DNA in plasma or cell-free circulating tumor DNA as well as Circulating Tumor Cells (CTC). The company’s proprietary liquid biopsy technology provides in-depth clinical information to physicians for monitoring and treating patients with cancer. The company also provides services to other laboratory testing providers, research organizations, academic institutions, biopharmaceutical firms, and clinical trial support organizations. The company has partnerships with several market leaders and major cancer centers, which, in turn, helps the company to advance in the field of cancer diagnostics.
-
BioCEP Ltd., The company focuses on developing cell isolation technologies. BioCEP’s patented Cell Enrichment Process is included in the Cell Enrichment Process for Isolation of Rare Cells (CEPir) devices for cell isolation to allow efficient cell isolation in various R&D applications. The company manufactures, develops, and commercializes products to customers globally.
-
Cell Microsystems (Fluxion Biosciences, Inc.), Cell Microsystems is a life sciences company developing innovative tools to revolutionize single-cell research. Their core technology, CellRaft, enables researchers to isolate, image, and analyze individual cells with unprecedented precision and efficiency. This empowers scientists in various fields like CRISPR gene editing, oncology, stem cell biology, immunology, and neurobiology to make groundbreaking discoveries. The company acquired Fluxion Biosciences, Inc. Fluxion Biosciences, Inc. focuses on the development of analytical instruments for functional cellular analyses. The company develops automated solutions for cell-free and cell-based assays.
-
Greiner Bio One International GmbH, Greiner Bio One International GmbH is an international medical technology company that operates as a subsidiary of Greiner BioOne International AG. This company operates through four business divisions—analytics, biosciences, diagnostics, and equipment manufacturing. Preanalytical products include venous blood collection, urine collection, capillary blood collection, and saliva collection systems. Moreover, the company offers customized platforms for life sciences and medical sectors as well as biochips for bacterial and viral identification.
-
Ikonisys Inc., Ikonisys, Inc. is involved in designing and manufacturing medical diagnostic products targeted toward cancer diagnostics. The products manufactured by the company help in early cancer diagnosis and genetic disorder screening. Ikonisys generates its revenue via three key business segments, including medical devices, services, and in vitro diagnostics products. Catering to customers operating in cell-based diagnostics, Ikonisys manufactures products that help in the efficient detection of rare cells.
-
Miltenyi Biotec, Miltenyi Biotec is a biotechnology company involved in cell-based research and its clinical translation, for which it provides advanced cellular therapy and biomedical research solutions. Its GMP and contract manufacturing facility is located in Teterow, Germany. The company supplies products in countries including the UK, France, Italy, Spain, Japan, China, Australia, Singapore, South Korea, and Australia. It also focuses on R&D activities pertaining to immunology, stem cell research, neuroscience, cancer research, and cardiovascular research.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Circulating Tumor Cells Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2023, historic information from 2018 to 2023, and forecast from 2024 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Circulating Tumor Cells Market Report Assumptions:
-
The report provides market value for the base year 2023 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





