- Home
- »
- Medical Devices
- »
-
Clinical Trial Site Management Organizations Market Report, 2030GVR Report cover
![Clinical Trial Site Management Organizations Market Size, Share & Trends Report]()
Clinical Trial Site Management Organizations Market Size, Share & Trends Analysis Report By Clinical Trail Service/Component, By Phase, By Therapeutic Areas (Oncology, Cardiology, CNS), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-4-68039-921-9
- Number of Report Pages: 275
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Market Size & Trends
The global clinical trial site management organizations market size was estimated at USD 6.24 billion in 2023 and is anticipated to grow at a compound annual growth rate (CAGR) of 6.17% from 2024 to 2030. The market has noticed critical growth on account of rising R&D investments by pharmaceutical companies owing to high burden of chronic and infectious diseases. Apart from this, the COVID-19 pandemic has contributed significantly to clinical trials demand, as of 12th April 2022, over 4,645 studies for COVID-19 were in the active stage. Robust dug pipeline and sponsors are key growth determinants of this market.
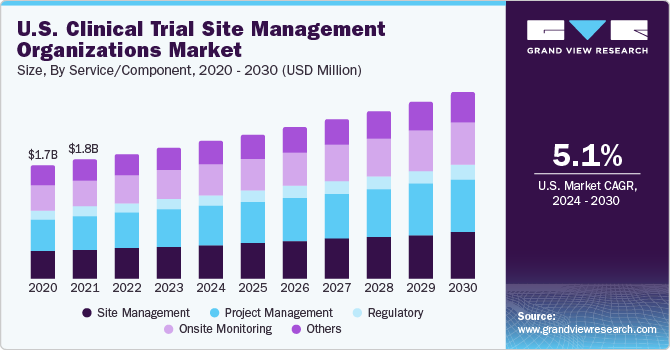
In recent years, the number of clinical trials has increased tremendously. According to Clinical trials.gov, the total number of clinical trials accounted for 325,773 in 2020, whereas, as of April 2022, the number of clinical trials accounted for 410,903. Rising clinical trial activities has profited the market for clinical trial site management organizations. Complexities associated with clinical trials have improved demand for CROs. A significant number of CROs offering clinical trial site management organizations solutions have further promoted market growth.
During the advent of the pandemic, clinical trials were halted to reduce spread of the COVID-19 virus. However, owing to increasing urgent need for vaccines, diagnostics, and therapeutics, clinical trials were resumed by following social distancing measures. Public organizations globally had made a high investment in R&D to support the clinical trials of COVID-19 vaccines and therapeutics. For instance, as of February 2022, the U.K government invested USD 119.1 million to support development of the Oxford/AstraZeneca COVID-19 vaccine. This is expected to have a positive impact on the clinical trial site management organizations market.
Clinical research sites have historically been a highly fragmented sector, consisting largely of part-time physician practices and small stand-alone companies. Consolidation has been occurring at an unprecedented rate, with sites coming together under common ownership or administration to operate as a single network. There are two business models in the clinical research industry for clinical trial sites: free-standing research sites, or dedicated research centers, and physician affiliated clinical trial sites.
Phase Insights
Based on phase, the phase III segment held a maximum revenue share of 54.16% in 2023 and is predicted to witness a similar trend during forecast years. Clinical studies in phase III are more complicated than earlier stages. This phase also includes a greater number of patients than any other phases which increases site management service demand. Moreover, failure rate is also highest in this phase as sample size and research design need precise dosing at optimal level. Such complications further promote segmental demand.
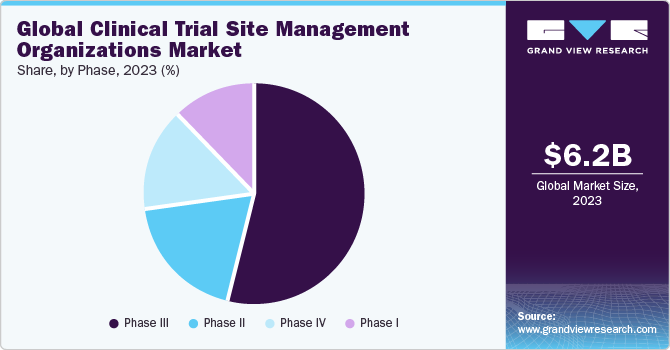
The phase I segment is anticipated to witness a fastest CAGR of 6.63% over the forecast period. An upsurge in R&D spending and increase in innovative treatments demand is contributing to phase I trials demand and thereby promoting need for site management services. High burden of diseases globally, contributing to the demand for new research is likely to accelerate impact on segment growth.
Regional Insights
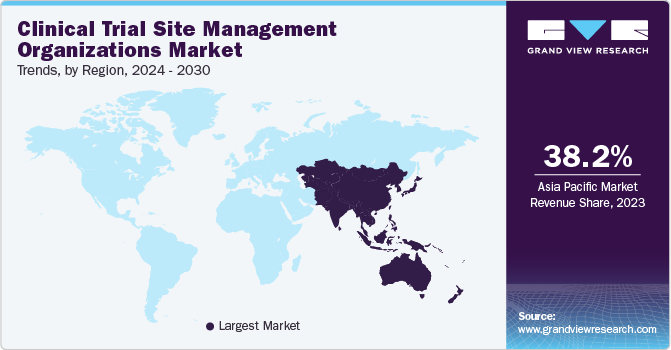
Asia Pacific is projected to witness a CAGR of 6.87% over the analysis time frame. The region has become a hotspot for conducting clinical trials owing to favorable regulatory compliance, growing patient population, cheap study costs, and existence of the several elite clinical institutions functioning as sites. Thus, above-mentioned factors are supporting site management services demand, thus promoting market growth.
Site management organizations operating as independent Contract Research Organizations (CROs) offer some significant advantages among the evolving Asian market for clinical trials where fast recruitment of huge numbers of patients is essential. They are typically used when district physicians or hospital physicians serve as investigators. The majority of study visits are overseen by the patient's personal doctor, who is assisted by practice/research nurses. Such SMOs would be in charge of site training as well as assistance and part of a regional coordination framework. Moreover, patients are recruited through doctor visits and database searches, making the model suited for large numbers of patient enrollment.
Market Dynamics
The expansion of clinical trials into emerging markets and development of novel therapies for numerous chronic diseases contributing to market growth. Furthermore, pharmaceutical companies outsource to SMOs to access specialized expertise and resources without maintaining an in-house infrastructure. This outsourcing trend allows sponsors to focus on core competencies such as drug development and marketing, while SMOs handle site management, patient recruitment, and regulatory compliance in managing clinical trial sites. In addition, pharmaceutical companies can scale their involvement in SMOs based on specific needs of each clinical trial. This flexibility is particularly valuable in the dynamic and variable landscape of clinical research. Thus, aforementioned factors positively influencing market growth.
Private equity and other institutional investors are more involved in clinical trial sites. The investment thesis for private equity companies offers opportunities to consolidate operations in a highly fragmented industry, resulting in a predictable cash-flow business with economies of scale. Many companies are actively pursuing freestanding sites as part of a roll-up strategy.They prefer to acquire sites with a minimum revenue size, a track record of predictable positive EBITDA and a diverse PI base, or, at very least, an active PI who agrees to stay on for an extended period of time. Other institutionally funded companies are pursuing a physician-only model. These companies are exploring relationships with a large number of medical practices in order to scale. Large health systems are particularly appealing partners due to their size and reach, both in terms of PIs and patients.
Service/Component Insights
Based on service/component, the project management segment held the largest revenue share of over 25.0% in 2023. A clinical trial is considered a large and complex project. Project management involves several complex components and moving parts. These include components such as study design, recruiting, labs, an investigational product, data, materials, and site management. Various project tools and templates can be utilized prior to starting a clinical trial to successfully design and execute a clinical trial. Several tools include Microsoft Access, Excel, SharePoint, Outlook, Visio, and Web-based applications.The clinical trial project management life cycle includes team management, study start-up, clinical evaluation, recruiting, and intervention.
The onsite monitoring segment is expected to witness the fastest CAGR of 6.62% throughout the forecast period. Site monitoring service records progress of clinical trials, and ensures that monitoring is conducted, and reported in accordance with SOPs of clinical studies. Clinical trials sites are required to be monitored as per the requirements of the GCP. Growing number of clinical trials have improved demand for onsite monitoring services in the market for clinical trial site management organizations.
Therapeutic Areas Insights
Based on therapeutic areas, the CNS segment dominated the market and accounted for a revenue share of 16.3% in 2023. High prevalence of CNS associated conditions is contributing to demand for clinical trials, thereby driving segmental growth. For instance, the WHO in 2023 reported that over 55.0 million people have dementia worldwide. Growing geriatric population is one of the key reasons for high prevalence of dementia. Increasing funding for clinical studies for CNS from various public organizations is expected to improve demand for site management and is likely to support segmental demand.
The oncology segment is anticipated to witness the fastest CAGR of 6.66% in the market during the forecast period. Cancer is one of the major causes of deaths worldwide. As per the estimates of Cancer Tomorrow (WHO), 30.2 million people are likely to have cancer till 2040. High prevalence of chronic diseases is contributing to its demand in research; this is the prime reason for segmental growth. Sedentary lifestyle is further contributing to cancer incidence which is expected to further promote diseases incidence thus promoting research demand for cancer trials.
Key Companies and Market Share Insights
Mergers, acquisitions, and partnerships among others were key strategies adopted by key players to maintain their market share. For instance, in April 2022, ClinChoice partnered witha clinical research and development solution provider Cloudbyz. Through this partnership, Cloudbyz provided its clinical research management technology platform for helping Clinichoice in clinical studies, with services such as site identification, site feasibility, and clinical monitoring. Similarly, in September 2021, Elligo Health acquired ClinEdge for USD 135 million to expand its clinical trials service offerings.
Key Clinical Trial Site Management Organizations Companies:
- Clinedge
- WCG
- ClinChoice
- Access Clinical Research
- FOMAT Medical Research INC.
- SGS
- KV Clinical
- SMO-Pharmina
- Xylem Clinical Research
- Aurum Clinical Research
Clinical Trial Site Management Organizations Market Report Scope
Report Attribute
Details
Market Size value in 2024
USD 6.6 billion
Revenue forecast in 2030
USD 9.47 billion
Growth rate
CAGR 6.17 % from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Report updated
November 2023
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Clinical trial services/components, phase, therapeutic areas, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Report coverage
Revenue forecast, company share, competitive landscape, growth factors and trends
Key companies profiled
Clinedge; WCG; ClinChoice; Access Clinical Research; FOMAT Medical Research INC.; SGS; KV Clinical; SMO-Pharmina; Xylem Clinical Research; Aurum Clinical Research
Customization scope
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional, and segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
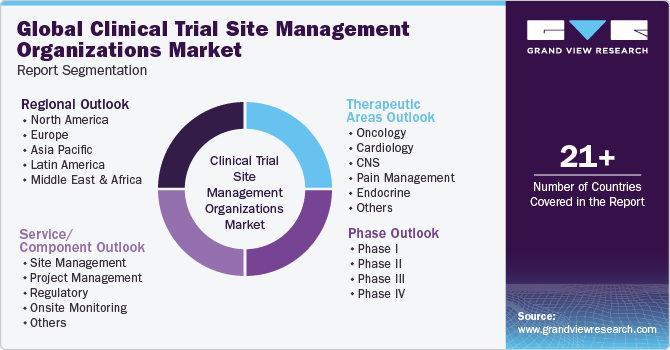
Global Clinical Trial Site Management Organizations Market Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 - 2030. For this study, Grand View Research has segmented the global clinical trial site management organizations market report based on clinical trial service/ component, phase, therapeutic areas, and region:
-
Services/ Component Outlook (Revenue, USD Million, 2018 - 2030)
-
Site Management
-
Project Management
-
Regulatory
-
Onsite Monitoring
-
Others
-
-
Phase Outlook (Revenue, USD Million, 2018 - 2030)
-
Phase I
-
Phase II
-
Phase III
-
Phase IV
-
-
Therapeutic Areas Outlook (Revenue, USD Million, 2018 - 2030)
-
Oncology
-
Cardiology
-
CNS
-
Pain Management
-
Endocrine
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
Colombia
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global clinical trials site management organizations market size was estimated at USD 6.24 billion in 2023 and is expected to reach USD 6.6 billion in 2024.
b. Key factors that are driving the clinical trials SMO market growth include an upsurge in the number of clinical studies along with rising investment in R&D by pharmaceutical companies.
b. The global clinical trials site management organizations market is expected to grow at a compound annual growth rate of 6.17% from 2024 to 2030 to reach USD 9.47 billion by 2030.
b. The Asia Pacific dominated the clinical trials site management organizations market with a share of 38.2% in 2023. This is attributable to the increasing rate of clinical research outsourcing across the region, due to its less expensive nature.
b. Some key players operating in the clinical trials site management organizations market include Clinedge, WCG, ClinChoice, Access Clinical Research, FOMAT Medical Research INC., SGS, KV Clinical, SMO-Pharmina; Xylem Clinical Research, and Aurum Clinical Research
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





