- Home
- »
- Medical Devices
- »
-
Endoscope Reprocessing Market Size, Industry Report 2033GVR Report cover
![Endoscope Reprocessing Market Size, Share & Trends Report]()
Endoscope Reprocessing Market (2026 - 2033) Size, Share & Trends Analysis Report By Product (High-Level Disinfectants and Test Strips, Detergents and Wipes, Automated Endoscope Reprocessors), By End Use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-265-5
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2021 - 2025
- Forecast Period: 2026 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Endoscope Reprocessing Market Summary
The global endoscope reprocessing market size was estimated at USD 1.80 billion in 2025 and is projected to reach USD 3.02 billion by 2033, growing at a CAGR of 6.79% from 2026 to 2033. Growing infections caused by contaminated endoscopes and increasing preference for minimally invasive surgery are anticipated to boost market growth.
Key Market Trends & Insights
- The North America endoscope reprocessing market dominated the global market in 2025, accounting for the largest revenue share of 37.84%.
- The Canada endoscope reprocessing market is anticipated to register the fastest growth rate during the forecast period.
- In terms of product, the high-level disinfectants and test strips segment held the largest revenue share in 2025.
- In terms of the end use, the ambulatory surgical centers (ASCs) segment held the largest revenue share in 2025.
Market Size & Forecast
- 2025 Market Size: USD 1.80 Billion
- 2033 Projected Market Size: USD 3.02 Billion
- CAGR (2026-2033): 6.79%
- North America: Largest market in 2025
- Asia Pacific: Fastest growing market
According to a study titled "Cannulation rates and technical performance evaluation of commercially available single-use duodenoscopes for endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis" published in January 2024, the range of infections related to duodenoscopes was between 0.4% and 1%.
The increasing burden of respiratory disease, primarily chronic obstructive pulmonary disease (COPD), is driving rapid market growth. According to the JAMA Network report, the global prevalence of COPD was estimated to be 10.6% in 2020, which equals 480 million cases across both genders. The report predicts that the number of cases is expected to rise by 112 million to 592 million by 2050, which is 9.5% of the population. This indicates a relative increase of 23.3% from 2020 to 2050. As a result, the demand for endoscopic devices is increasing significantly for early disease diagnosis and treatment, which is further propelling the market growth.
Cervical Cancer in the U.S. (2025)
Metric
Value (2025 Estimate)
Annual new cases (invasive)
13,360
Annual deaths
Approximately 4,320
Median age at diagnosis
~ 50 years
Incidence proportion of total cancers
~ 0.7%
Age-adjusted incidence rate (women)
~ 7.7 per 100,000 women/year
Source: U.S. Department of Health and Human Services & GVR
Furthermore, the convergence of endoscopy and robotics has revolutionized surgical possibilities, providing improved visualization, unparalleled precision, and better patient outcomes. For instance, the endoscopic surgical system from Virtuoso features two needle-sized manipulators that are robotically controlled, operating from the tip of an endoscope to bring the stability and precision of robot-assisted surgery to rigid endoscopy. Moreover, an increasing number of successful clinical trials is expected to boost product approvals. In January 2023, Agilis Robotics, a prominent developer of flexible robotic instruments, completed the second round of live animal testing with its proprietary robot for endoscopic surgery. The test results were satisfactory and demonstrated promising outcomes for the firm's medical robotic system, including its efficacy, accuracy, and safety. Thus, increasing integration of robotics in endoscopic surgery will lead to a rise in the number of procedures, thereby driving market growth.
Rising funding and investments in R&D activities for the development of improved endoscope products are expected to drive the growth of the endoscope reprocessing market. In January 2023, IQ Endoscopes, a medical device company, secured a USD 6.6 million investment in a funding round led by BGF. The investment will be used to support the development and launch of IQ Endoscope’s medical device, designed for the early diagnosis of various cancers and other gastrointestinal conditions. Thus, the increasing launch of endoscopy devices will drive the growth of endoscope reprocessing.
Furthermore, regulatory bodies such as the FDA (Food and Drug Administration) and CDC (Centers for Disease Control and Prevention) have established guidelines and standards for reprocessing endoscopes to reduce the risk of infections associated with endoscopic procedures. Adherence to these regulations is a significant driver for adopting advanced endoscope reprocessing systems and solutions. In April 2022, the Food and Drug Administration issued a recall and instructed healthcare providers to use different reprocessing methods for certain urological endoscopes manufactured by Karl Storz.
Market Concentration & Characteristics
The endoscope reprocessing industry has seen significant innovation in recent years, due to technological advances, stringent regulatory requirements, and concerns about infection control. There has been a shift towards developing automated reprocessing systems with advanced features to improve efficiency and reduce the risk of human error. These systems use cutting-edge technologies, such as robotics, artificial intelligence (AI), and advanced sensors, to streamline the reprocessing workflow and minimize the potential for contamination.
The market is characterized by the leading players' high level of merger and acquisition (M&A) activity. This is due to several factors, including the desire to expand the business to cater to the growing demand for endoscope reprocessing. In November 2023, HOYA Corporation acquired WASSENBURG Medical B.V, a global manufacturer of endoscope reprocessing systems, consumables, and services.
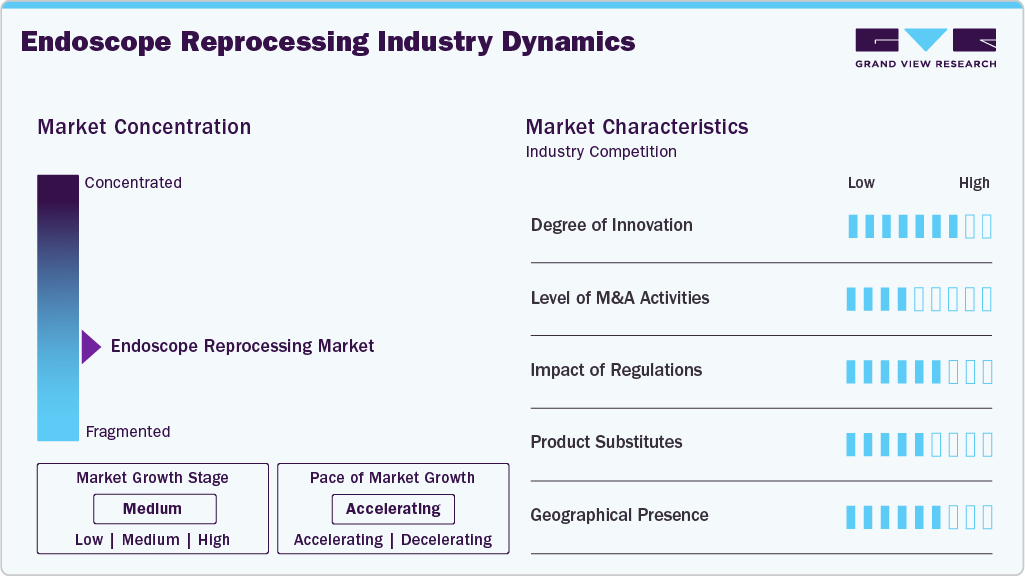
Regulations are crucial in shaping the market for endoscope reprocessing, as they ensure patient safety and maintain the efficacy of medical devices. Endoscopes are essential tools in modern healthcare for diagnosing and treating a wide range of medical conditions. However, their intricate design and direct contact with bodily fluids pose significant challenges for effective cleaning and disinfection, necessitating stringent regulations.
Product substitutes refer to alternative methods or technologies that healthcare facilities can use instead of traditional reprocessing procedures. One notable substitute in the market is single-use, disposable endoscopes. These devices are designed for one-time use and eliminate the need for reprocessing.
Several market players are expanding their business by entering new geographical regions to strengthen their market position and product portfolio. Rising product launches create more opportunities for market players to enter new areas. In February 2025, Nanosonics continued to advance its strategic product expansion efforts, focusing on ultrasound and endoscope reprocessing technologies and digital health innovations. During this period, the company allocated USD 16.4 million towards research and development, reflecting a 1% increase from the previous year. Notably, around two-thirds of this R&D investment was dedicated to developing CORIS, the company’s upcoming endoscope reprocessing platform, highlighting its commitment to innovation in infection prevention.
Product Insights
By product, the high-level disinfectants and test strips segment dominated the market in 2025, accounting for the largest revenue share of 29.73%. The healthcare industry is focusing more on preventing and controlling infections in healthcare facilities, which is leading to market growth. Healthcare-associated infections (HAIs) remain a significant threat to patient safety, prompting healthcare facilities to prioritize the implementation of stringent disinfection protocols for endoscopes. An NIH article published in 2022 reports that after GI endoscopic procedures, the composite infection rate was found to be 0.2%, while it was 0.8% following Endoscopic retrograde cholangiopancreatography (ERCP), 0.123% following non-ERCP upper GI endoscopic procedures, and 0.073% following lower GI endoscopic procedures. High-level disinfectants are crucial in effectively eliminating microorganisms, such as bacteria, viruses, and fungi, from endoscopic instruments. This helps reduce the risk of cross-contamination and the transmission of infectious agents between patients, thereby supporting segmental growth.
The automated endoscope reprocessors (AERs) segment is anticipated to witness the fastest CAGR over the forecast period. Technological advancements in AERs contribute to market growth. Manufacturers continuously improve AERs to enhance their functionality, efficiency, and safety features. Advanced AERs are equipped with high-level disinfection cycles, endoscope-specific adapters, and compatibility with a wide range of endoscope models. For instance, ASP's AEROFLEX AER is an automated system designed explicitly for endoscope reprocessing. The AER comes with an integrated minimum recommended concentration (MRC) monitor. This eliminates the need for test strips. The AER has a complete cycle time of only 22 minutes, making it a highly efficient solution for endoscope reprocessing.
End Use Insights
The Ambulatory Surgical Centers (ASCs) segment dominated the market in 2025 with a revenue share of 56.96%. Outpatient settings, including ASCs, are witnessing a surge in the volume of endoscopic procedures, thereby escalating segmental growth. According to an article published by McMahon Publishing in 2024, the number of surgical procedures performed at ASCs is expected to increase by at least 25% in the next 10 years. Moreover, the integration of artificial intelligence (AI) and digitalization into the endoscopy procedure is driving segmental growth. In January 2024, Omega Healthcare and Sanjivani Gastro Liver Clinic introduced AI-enabled endoscopy in Odisha, India, to aid in the diagnosis and treatment of gastrointestinal disorders.
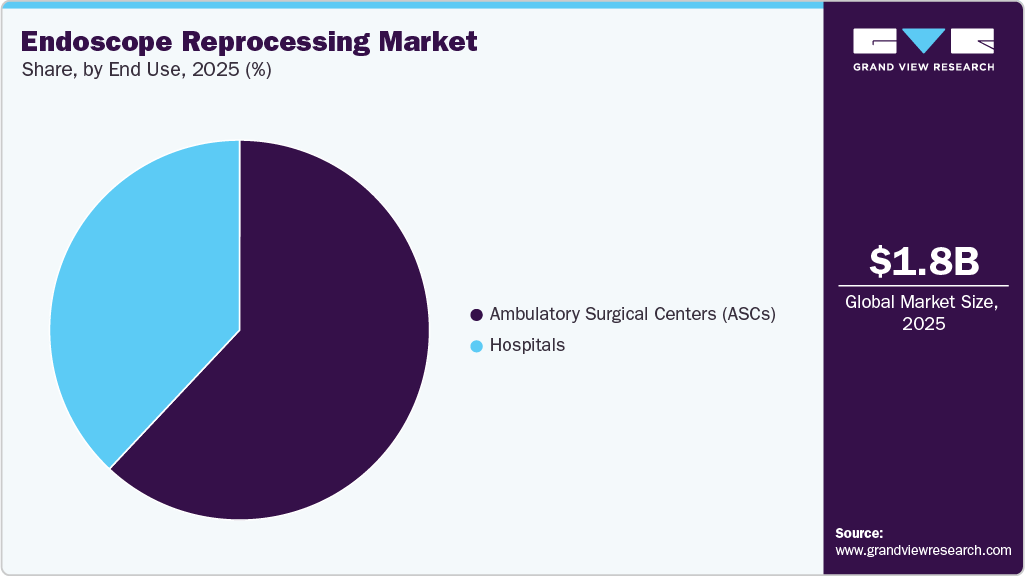
The hospitals segment is anticipated to register a significant growth rate over the forecast period. Hospitals play a crucial role in providing medical procedures, diagnostics, and treatments, including a diverse range of endoscopic procedures. To ensure high standards of patient care and infection prevention, hospitals require reliable endoscope reprocessing solutions. In addition, the rising prevalence of chronic diseases and the rising patient preference for hospitals drive market growth in this area.
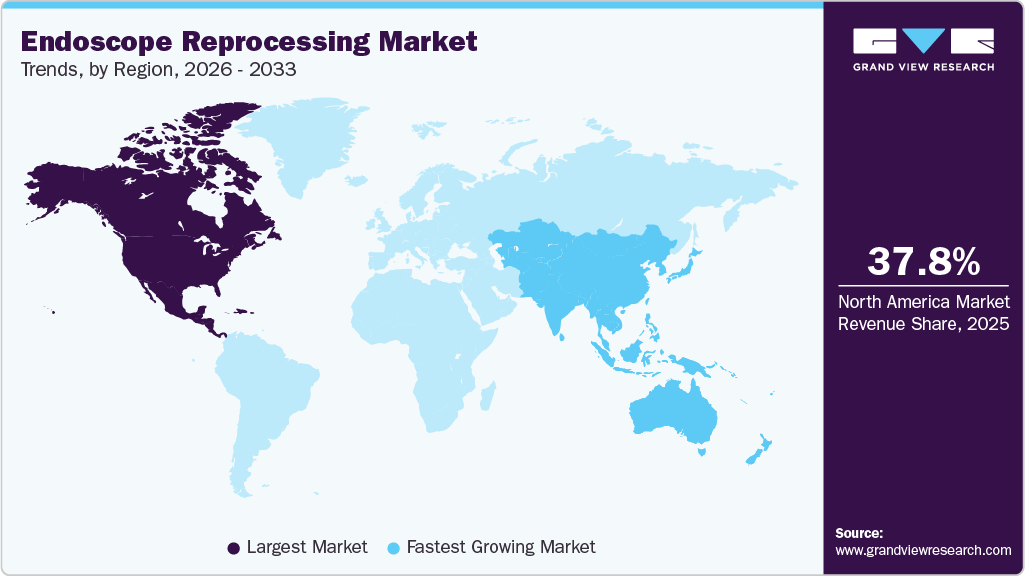
Regional Insights
The North America endoscope reprocessing industry dominated the market in 2025, accounting for the largest revenue share of 37.84% due to a well-established healthcare infrastructure and high healthcare expenditure. Besides, the increasing prevalence of gastrointestinal diseases, cancer, and other medical conditions requiring endoscopic procedures contributes to the growth in North America. For instance, according to the GI Alliance report, 62 million Americans are diagnosed with digestive disorders each year.
U.S. Endoscope Reprocessing Market Trends
The endoscope reprocessing industry in the U.S. held the largest share, 82.37%, in 2025. Increasing cases of gastrointestinal diseases and the high volume of endoscopic procedures performed annually are driving the market growth. For instance, according to the ResearchGate article, in the U.S., more than 17.7 million gastrointestinal (GI) endoscopic procedures are performed every year, making up 68% of all endoscopic procedures.
The Canada endoscope reprocessing industry is anticipated to register the fastest growth rate during the forecast period. Growing demand for surgeries with fewer post-surgery infections, faster recovery, reduced scarring, better control of bleeding, and less pain is expected to boost the demand for endoscope devices, thereby fostering market growth.
Europe Endoscope Reprocessing Market Trends
The endoscope reprocessing industry in Europe is poised to register significant growth during the forecast period. Growing product launches and approvals in the region are accelerating the market growth. In June 2023, PENTAX Medical Europe, a part of the HOYA Group, introduced a brushless automated pre-cleaning solution for endoscopes. This new solution is called AquaTYPHOON.
The Germany endoscope reprocessing industry is anticipated to register a considerable growth rate during the forecast period. A growing number of endoscopy procedures and increasing technological advancements in the field of endoscopy devices are anticipated to escalate market growth.
The endoscope reprocessing industry in the UK is expected to register a considerable growth during the forecast period. Rising investment from public and private investors in endoscopy services will escalate market growth. In October 2023, Clearview Endoscopy Limited, a company that provides maintenance, repair, and servicing of flexible endoscopes used for medical diagnosis in healthcare settings, received an investment of USD 7.7 million from Foresight.
The Spain endoscope reprocessing industry is anticipated to exhibit a significant growth rate during the forecast period. Increased prevalence of cancer, such as lung and stomach cancer, is boosting market growth. The rising number of colorectal cancer cases in Spain has driven the growth of the market. According to the Spanish Network of Cancer Registries article published in March 2025, an estimated 44,500 new cases are projected in 2025. The disease shows a higher prevalence among men, with a ratio of three male cases for every two in women. In spite of a stable incidence trend, colorectal cancer continues to be the second leading cause of cancer-related deaths in the country, accounting for over 15,000 fatalities in 2022—approximately 13.7% of all cancer deaths. This significant burden highlights the vital role of early detection and intervention through endoscopic procedures.
Asia Pacific Endoscope Reprocessing Market Trends
The endoscope reprocessing industry in the Asia Pacific is anticipated to be the fastest-growing region in the market, owing to its rapidly improving healthcare infrastructure, a less stringent regulatory framework, and economic development attracting foreign investments. Key market players are developing strategies to expand their business in this region. Market growth is expected to be driven by favorable initiatives undertaken by private players, such as training healthcare professionals and increasing R&D investments to develop advanced endoscopes.
The Japan endoscope reprocessing industry is anticipated to register a significant growth rate during the forecast period. The growing prevalence of chronic diseases and consistent product advancements are some factors expected to drive the demand for endoscopy procedures, further propelling the market growth. For instance, in July 2024, Olympus announced its aim to evolve from an endoscope manufacturer to a diagnostic platform provider by leveraging AI to assist doctors in performing treatments. The Japanese company plans to launch a trial program in Europe this year to evaluate a cloud-based service connecting hospital endoscopes to its cloud-based software platform.
The endoscope reprocessing industry China is anticipated to register a significant growth rate during the forecast period. Increasing collaborations among market players to develop advanced endoscopes is one of the key factors driving market growth. For instance, in July of 2023, HOYA Group and PENTAX Medical announced the opening of a new facility in Shanghai, China. This center would focus on manufacturing, R&D, and servicing endoscopes for the Chinese market under PENTAX Medical Shanghai Co., Ltd. The facility would produce PENTAX Medical’s endoscopic solutions tailored to the Chinese market.
The India endoscope reprocessing industry is anticipated to register a significant growth rate during the forecast period. The Union Minister for Health and Family Welfare noted that the number of cancer cases in India was 1,496,972 in 2023, compared to 1,461,427 in 2022. As per an article released by the Press Information Bureau (PIB) in 2023, the nation implemented a population-centered program for the prevention, management, and screening of prevalent Non-Communicable Diseases (NCDs) such as hypertension, diabetes, and common types of cancer under the National Health Mission (NHM) and as part of Comprehensive Primary Health Care. The program aims to screen individuals over 30 for the three common cancers: breast, oral, and cervical. These initiatives aim to increase awareness and enable early cancer diagnosis, fueling market growth.
Latin America Endoscope Reprocessing Market Trends
The endoscope reprocessing industry in Latin America is expected to grow over the forecast period, driven by the increasing shift toward minimally invasive procedures and the rising awareness of the importance of endoscopy in both diagnostic and therapeutic applications. This growing preference contributes to a greater demand for safe and effective reprocessing systems across the region.
The Brazil endoscope reprocessing industry is growing over the forecast period. Brazil, the largest economy in Latin America, represents a high-growth potential market for medical devices, including endoscope reprocessing systems. The country's increasing geriatric population and the rising prevalence of chronic diseases such as gastrointestinal (GI) disorders, cardiac valve conditions, sinusitis, and gynecological issues are key drivers of market expansion. According to the Brazilian National Cancer Institute (INCA), Brazil is projected to record approximately 704,000 new cancer cases between 2023 and 2025, underscoring the growing demand for diagnostic and therapeutic procedures, such as endoscopy.
Middle East and Africa Endoscope Reprocessing Market Trends
The endoscope reprocessing industry in the Middle East and Africa (MEA) is growing over the forecast period. The MEA is an economically diverse yet rapidly developing region, with certain countries such as those in the Gulf Cooperation Council (GCC) boasting high per capita disposable incomes and technologically advanced healthcare systems. Government initiatives to expand reimbursement coverage are expected to boost market growth further over the forecast period.
The South Africa endoscope reprocessing industry is growing notably over the forecast period. Increasing colorectal cancer cases drive the market's growth, according to Cansa.org. In April 2024, in South Africa, colorectal cancer represents a significant health concern, with 2022 data from the National Cancer Registry indicating 2,467 newly diagnosed cases in men and 2,221 in women, all confirmed through histological analysis. This equates to a lifetime risk of approximately 1 in 76 for males and 1 in 124 for females, accounting for about 5.9% of all male cancers and 4.85% of all female cancers in the country. The growing incidence of colorectal cancer is driving an increased demand for timely diagnostic procedures such as colonoscopy, which in turn is boosting the need for efficient endoscope reprocessing solutions.
Key Endoscope Reprocessing Company Insights
Key participants in the global market are focusing on devising innovative business growth strategies in the form of product portfolio expansions, partnerships & collaborations, mergers & acquisitions, and business footprint expansions.
Key Endoscope Reprocessing Companies:
The following are the leading companies in the endoscope reprocessing market. These companies collectively hold the largest Market share and dictate industry trends.
- Cantel Medical
- Fortive Corporation (Advanced Sterilization Products)
- Olympus Corporation
- Ecolab
- Getinge AB
- STERIS
- Steelco S.p.A
- ARC Group of Companies Inc.
- Metrex Research, LLC.
- Shinva Medical Instrument Co. Ltd.
- Belimed
Recent Developments
-
In May 2025, Olympus Corporation introduced the ScopeLocker Air, a specialized endoscope drying cabinet developed to support proper drying and storage protocols in medical facilities. Designed to aid in the final stage of the endoscope reprocessing cycle, this system effectively dries internal channels, aligning with best practice guidelines from health authorities. Manufactured by Capsa Healthcare and offered through Olympus, the cabinet provides a dependable and compliant drying solution to enhance patient safety and streamline the handling of endoscopes.
-
In February 2025, Nanosonics continued to advance its strategic product expansion efforts, focusing on ultrasound and endoscope reprocessing technologies and digital health innovations. During this period, the company allocated USD 16.4 million towards research and development, reflecting a 1% increase from the previous year. Notably, around two-thirds of this R&D investment was dedicated to developing CORIS, the company’s upcoming endoscope reprocessing platform, highlighting its commitment to innovation in infection prevention.
-
In September 2024, Soluscope and its manufacturing site achieved compliance with the EU Medical Device Regulation (MDR 2017/745), enabling the launch of CE‑marked products such as the Serie XS automated endoscope reprocessor.
Endoscope Reprocessing Market Report Scope
Report Attribute
Details
Market size value in 2026
USD 1.91 billion
Revenue forecast in 2033
USD 3.02 billion
Growth Rate
CAGR of 6.79% from 2026 to 2033
Actual data
2021 - 2025
Forecast data
2026 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2026 to 2033
Report coverage
Revenue & Volume forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East and Africa
Country scope
U.S.; Canada; Mexico; Germany; UK; Spain; Italy; France; Norway; Denmark; Sweden; Japan; China; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Cantel Medical; Fortive Corporation (Advanced Sterilization Products); Olympus Corporation; Ecolab; Getinge AB; STERIS; Steelco S.p.A; ARC Group of Companies Inc.; Metrex Research, LLC.; Shinva Medical Instrument Co. Ltd.; Belimed
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
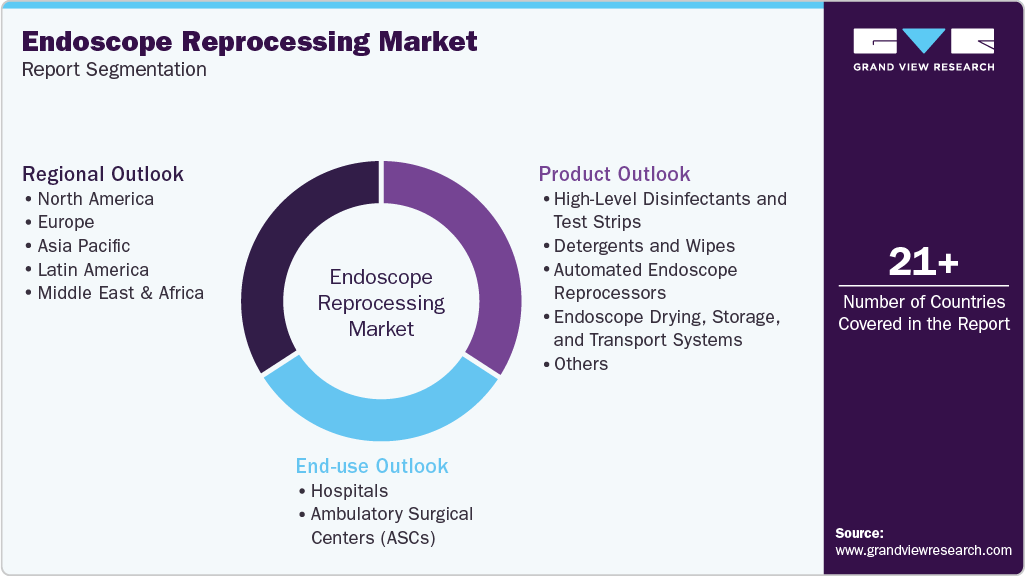
Global Endoscope Reprocessing Market Report Segmentation
This report forecasts revenue and volume growth at global, regional, and country levels and analyzes industry trends in each sub-segments from 2021 to 2033. For this study, Grand View Research, Inc. has segmented the global endoscope reprocessing market report based on product, end use, and region:
-
Product Outlook (Revenue, USD Million, 2021 - 2033)
-
High-Level Disinfectants and Test Strips
-
Detergents and Wipes
-
Automated Endoscope Reprocessors
-
By Type
-
Single basin
-
Double basin
-
-
By Portability
-
Standalone
-
Portable
-
-
-
Endoscope Drying, Storage, and Transport Systems
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2021 - 2033)
-
Hospitals
-
Ambulatory Surgical Centers (ASCs)
-
-
Regional Outlook Revenue, USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
Spain
-
Italy
-
France
-
Denmark
-
Norway
-
Sweden
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global endoscope reprocessing market size was estimated at USD 1.80 billion in 2025 and is expected to reach USD 1.91 billion in 2026.
b. The global endoscope reprocessing market is expected to grow at a compound annual growth rate of 6.79% from 2026 to 2033 to reach USD 3.02 billion by 2033.
b. High-level disinfectants and test strips dominated the market with a share of 29.73% in 2025. The healthcare industry is focusing more on preventing and controlling infections in healthcare facilities, which is leading to market growth. Healthcare-associated infections (HAIs) continue to be a significant threat to patient safety, so healthcare facilities are prioritizing the implementation of strict disinfection protocols for endoscopes.
b. Some key players operating in the market include Cantel Medical, Fortive Corporation (Advanced Sterilization Products), Olympus Corporation, Ecolab, Getinge AB, STERIS, Steelco S.p.A, ARC Group of Companies Inc., Metrex Research, LLC., Shinva Medical Instrument Co. Ltd., and Belimed
b. Growing infections caused by contaminated endoscopes and increasing preference for minimally invasive surgery are anticipated to boost market growth.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










