- Home
- »
- Pharmaceuticals
- »
-
Hunter Syndrome Treatment Market Size, Share Report 2030GVR Report cover
![Hunter Syndrome Treatment Market Size, Share & Trends Report]()
Hunter Syndrome Treatment Market Size, Share & Trends Analysis Report By Type (Enzyme Replacement Therapy, Hematopoietic Stem Cell Transplant), By Route Of Administration, By End Use, By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-2-68038-743-8
- Number of Report Pages: 100
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Hunter Syndrome Treatment Market Trends
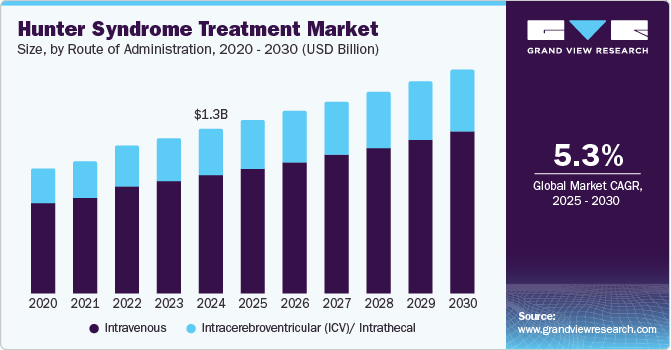
The global hunter syndrome treatment market size was estimated at USD 1.31 billion in 2024 and is projected to grow at a CAGR of 5.3% from 2025 to 2030. This growth is attributed to the increasing awareness of the disease and available treatments to enhance diagnosis and patient management, supported by initiatives such as the #FlyforMPS campaign. In addition, rising research and development expenditure fosters innovation in therapies, particularly enzyme replacement and gene therapies. Furthermore, government funding for rare disease treatments and favorable regulatory environments promote drug development, fueling market growth.
Hunter syndrome is a rare genetic disorder primarily affecting males. It arises from a deficiency of the enzyme iduronate-2-sulfatase, which is crucial for breaking down specific sugar molecules called glycosaminoglycans. When this enzyme is absent or malfunctioning, these sugars accumulate in various organs and tissues, leading to significant damage over time. This buildup can severely impact both physical and cognitive development.
Awareness about Hunter syndrome is on the rise, significantly impacting market growth. Campaigns such as #FlyforMPS, launched by Shire PLC in collaboration with organizations such as the International MPS Network and National MPS Society, have highlighted the condition's prevalence, affecting approximately one in 25,000 births in the U.S.
The surge in research and development activities further propels market opportunities. Companies are increasingly investing in innovative treatments, such as Shire's investigational therapy SHP609, which addresses cognitive impairments in pediatric patients. Moreover, funding from institutions such as the National Institute of Neurological Disorders and Stroke has facilitated advancements in gene therapy, which shows promise in halting disease progression.
In addition, increased financial support for drug approvals and health insurance coverage for rare diseases also drive pharmaceutical investments. These factors collectively create a conducive environment for developing effective therapies for Hunter syndrome despite challenges such as high treatment costs and a shortage of skilled healthcare professionals. The growing focus on rare diseases underscores the urgency for improved treatment options and patient outcomes.
Route Administration Insights
The intravenous segment led the market and accounted for the largest revenue share of 72.3% in 2024 attributed to its established effectiveness in delivering enzyme replacement therapies (ERT). This method allows for rapid distribution of therapeutic agents throughout the body, ensuring that essential enzymes reach target tissues effectively. Furthermore, healthcare professionals well-understood the IV route, facilitating easier administration and monitoring. As research continues to validate the effectiveness of ERT in managing physical symptoms, the IV route is likely to maintain its prominence in treatment protocols.
The intracerebroventricular (ICV)/intrathecal segment is expected to grow at a CAGR of 5.4% over the forecast period, owing to its nature of addressing neurological symptoms. These methods enable direct delivery of therapeutic agents into the central nervous system, overcoming barriers that limit traditional intravenous therapies. By targeting the brain and spinal cord directly, ICV and intrathecal routes enhance drug bioavailability and efficacy, especially for cognitive impairments associated with the disorder. Ongoing research and clinical trials further support these innovative approaches, making them increasingly attractive options for comprehensive patient care.
Type Insights
Enzyme replacement therapy (ERT) led the market and accounted for the largest revenue share of 57.3% in 2024 driven by its ability to address the deficiency of the enzyme iduronate-2-sulfatase. This therapy has been in use since 2006 and has shown significant clinical efficacy in managing the disease's somatic symptoms, such as organ enlargement. In addition, the increasing availability of ERT and growing awareness among healthcare professionals and patients are driving its adoption.
Hematopoietic stem cell transplant (HSCT) is expected to grow at a CAGR of 6.0% over the forecast period. Hematopoietic stem cell transplant (HSCT) is emerging as a promising treatment for Hunter syndrome, particularly for patients with severe forms of the disease. This approach aims to provide a long-term solution by replacing defective cells with healthy ones, potentially correcting the underlying genetic defect. The growing interest in HSCT is fueled by advancements in transplantation techniques and increased clinical trials demonstrating its effectiveness. As awareness of this treatment option expands, along with its potential to improve neurological outcomes, more families are considering HSCT as a viable alternative to traditional therapies, driving its growth in the market.
End Use Insights
Hospitals held the dominant position in the market, accounting for the largest revenue share of 63.1% in 2024 attributed to their capacity to provide comprehensive care and advanced medical resources. Hospitals have specialized staff and facilities that can administer complex therapies such as enzyme replacement therapy (ERT) and hematopoietic stem cell transplant (HSCT). This setting ensures that patients receive timely and effective treatment, which is crucial for managing the symptoms of Hunter syndrome. Hospitals often participate in clinical trials, contributing to ongoing research and innovation in treatment options, further enhancing their role in patient care.
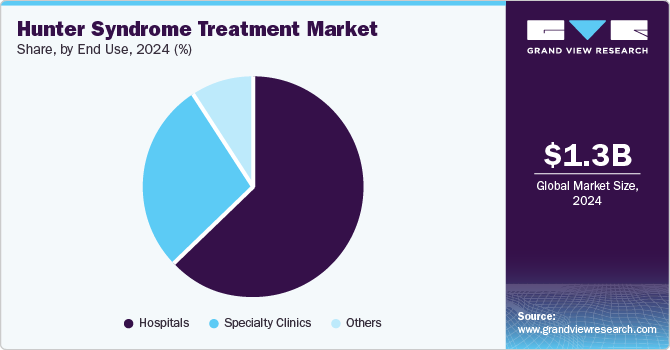
Specialty clinics are expected to grow significantly over the forecast period, owing to their focused expertise and personalized care. These clinics often employ healthcare professionals specializing in rare diseases, allowing for tailored treatment plans that address each patient's unique needs. In addition, the rise in awareness about Hunter syndrome has led to more patients seeking specialized care, driving demand for these clinics. Furthermore, specialty clinics typically offer a supportive environment for families navigating this complex condition, enhancing patient outcomes through dedicated resources and education.
Regional Insights
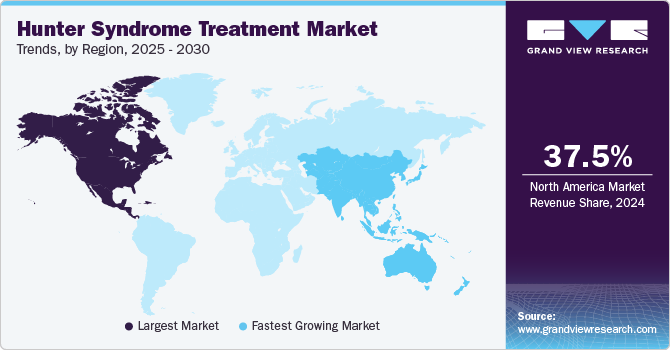
North America hunter syndrome treatment market dominated the global market and accounted for the largest revenue share of 37.5% in 2024 attributed to a combination of advanced healthcare infrastructure and increasing awareness of rare diseases. The presence of specialized treatment centers and expert medical professionals facilitates timely diagnosis and effective management of Hunter syndrome. Additionally, favorable reimbursement policies for expensive therapies, such as enzyme replacement therapy, encourage patient access to necessary treatments. Ongoing research and development initiatives further enhance the availability of innovative therapies, contributing to the market's growth in this region.
U.S. Hunter Syndrome Treatment Market Trends
Hunter syndrome treatment in the U.S. dominated the North American market and accounted for the largest revenue share in 2024 driven by significant investments in research and development. The country boasts a strong focus on orphan drug development, supported by government initiatives that promote innovation in rare disease therapies. Increased public awareness campaigns have led to earlier diagnoses and better patient outcomes. Furthermore, the availability of established therapies such as Elaprase and emerging treatments from biopharmaceutical companies ensures that patients can access effective management options, fueling market expansion.
Asia Pacific Hunter Syndrome Treatment Market Trends
The Asia Pacific hunter syndrome treatment market is expected to grow at the fastest CAGR of 6.3% over the forecast period, owing to rising awareness and improved medical education. Countries such as Japan and China are witnessing increased diagnosed cases, leading to heightened demand for effective therapies. The introduction of novel treatments, such as Hunterase, is expected to fill existing gaps in care. Furthermore, ongoing government initiatives to enhance healthcare infrastructure further support the expansion of Hunter syndrome treatment options across the region.
Hunter syndrome treatment market in China held a significant revenue share in Asia Pacific market attributed to increased diagnosed cases and a growing focus on rare diseases. Recent efforts to improve healthcare access and raise awareness about Hunter syndrome have resulted in more patients seeking treatment. Introducing innovative therapies such as Hunterase offers new hope for effectively managing the condition. As healthcare infrastructure continues to develop, the market is expected to expand rapidly, addressing unmet needs in this population.
Europe Pacific Hunter Syndrome Treatment Market Trends
Europe hunter syndrome treatment market is expected to witness substantial growth over the coming years, owing to increased awareness among healthcare professionals about rare diseases, which has led to more accurate diagnoses and timely interventions. In addition, strong regulatory frameworks support the approval of new therapies, while collaborations between pharmaceutical companies and research institutions enhance treatment options available to patients.
The growth of the hunter syndrome treatment market in Germany is driven by a strong emphasis on research and development within its advanced healthcare system. The country has a robust network of specialized clinics that provide comprehensive care for patients with rare diseases. In addition, increased funding for orphan drug research and favorable regulatory conditions facilitate the introduction of innovative therapies. Furthermore, growing public awareness campaigns have led to earlier diagnoses and improved patient outcomes, driving demand for effective treatments in Germany's healthcare landscape.
Key Hunter Syndrome Treatment Company Insights
Some key players in the market include Takeda Pharmaceutical Company Limited, F. Hoffmann-La Roche Ltd, Abbott, Medtronic, and others. These companies adopt strategies, including collaborations and partnerships, to stay competitive advantage. Furthermore, new product launches are pivotal; for instance, REGENXBIO’s RGX-121 has garnered attention for its potential in treating the condition. These strategies, combined with ongoing research and development efforts, drive innovation and improve treatment options for patients with Hunter syndrome.
-
Abbott develops innovative medical Enzyme Replacement Therapy (ERT), diagnostics, nutrition products, and branded generic pharmaceuticals. The company produces diagnostic tools that aid in the early detection and management of rare diseases. Their extensive portfolio includes advanced laboratory instruments and tests that facilitate accurate diagnosis, enabling healthcare providers to implement timely treatment strategies for patients with Hunter syndrome.
-
Medtronic specializes in developing Enzyme Replacement Therapy (ERT) and therapies for various medical conditions, including those affecting the cardiovascular, diabetes, and neurological segments. The company’s expertise in advanced drug delivery systems can be crucial in administering therapies such as enzyme replacement therapy. The company is committed to enhancing patient outcomes through innovative solutions that improve the delivery and efficacy of treatments for rare diseases such as Hunter syndrome.
Key Hunter Syndrome Treatment Companies:
The following are the leading companies in the hunter syndrome treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Takeda Pharmaceutical Company Limited
- F. Hoffmann-La Roche Ltd
- Abbott
- Denali Therapeutics
- Medtronic
- Johnson & Johnson Services, Inc.
- GSK Plc.
- Bayer AG
- Zimmer Biomet
- Stryker Corporation
- Homology Medicines, Inc.
- Novartis AG
Recent Developments
-
In September 2024, Denali Therapeutics announced plans to seek accelerated FDA approval for its investigational treatment, DNL310, to address Hunter syndrome (MPS II). Following productive discussions with the FDA, the company is set to submit a Biologics License Application (BLA) by early 2025. Interim data from ongoing clinical trials show significant improvements in key disease biomarkers and clinical outcomes, including cognitive and behavioral aspects. DNL310 employs an innovative enzyme transport vehicle designed to cross the blood-brain barrier, targeting both physical and neurocognitive symptoms of Hunter syndrome.
Hunter Syndrome Treatment Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 1.38 billion
Revenue forecast in 2030
USD 1.78 billion
Growth rate
CAGR of 5.3% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD Billion/Million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, route of administration, end use, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Germany, UK, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, Brazil, Argentina, Saudi Arabia, UAE, Kuwait, South Africa.
Key companies profiled
Takeda Pharmaceutical Company Limited; Denali Therapeutics; F. Hoffmann-La Roche Ltd; Abbott; Medtronic; Johnson & Johnson Services, Inc.; GSK Plc.; Bayer AG; Zimmer Biomet; Stryker Corporation; Homology Medicines, Inc.; Novartis AG.
Customization scope
Free report customization (equivalent to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Hunter Syndrome Treatment Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and analyzes the latest industry trends in each sub-segment from 2018 to 2030. For this study, Grand View Research has segmented the global hunter syndrome treatment market report based on type, route of administration, end use, and region.
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Enzyme Replacement Therapy (ERT)
-
Hematopoietic Stem Cell Transplant (HSCT)
-
Others
-
-
Route of Administration Outlook (Revenue, USD Million, 2018 - 2030)
-
Intravenous
-
Intracerebroventricular (ICV)/ Intrathecal
-
-
End use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Specialty Clinics
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





