- Home
- »
- Medical Devices
- »
-
Microelectronic Medical Implants Market Size Report, 2030GVR Report cover
![Microelectronic Medical Implants Market Size, Share & Trends Report]()
Microelectronic Medical Implants Market (2025 - 2030) Size, Share & Trends Analysis Report By Product (Pacemakers & Defibrillators, Neurostimulators), By Technology (RF Technology, Sensors), By Region And Segment Forecasts
- Report ID: GVR-2-68038-318-8
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
The global microelectronic medical implants market size was estimated at USD 34.14 billion in 2024 and is projected to reach USD 50.18 billion by 2030, growing at a CAGR of 6.6% from 2025 to 2030. The increasing prevalence of epilepsy, cardiac disorders, and Parkinson’s disease, rising geriatric population, growing number of regulatory approvals for new devices, and launch of new products are key factors driving market growth. Growing incidence of cardiac disorders is playing a key role in the market for microelectronic medical implants. Cardiovascular diseases (CVDs) such as cardiac arrest, congestive heart failure, stroke, and coronary artery diseases often require implants such as pacemakers and defibrillators.
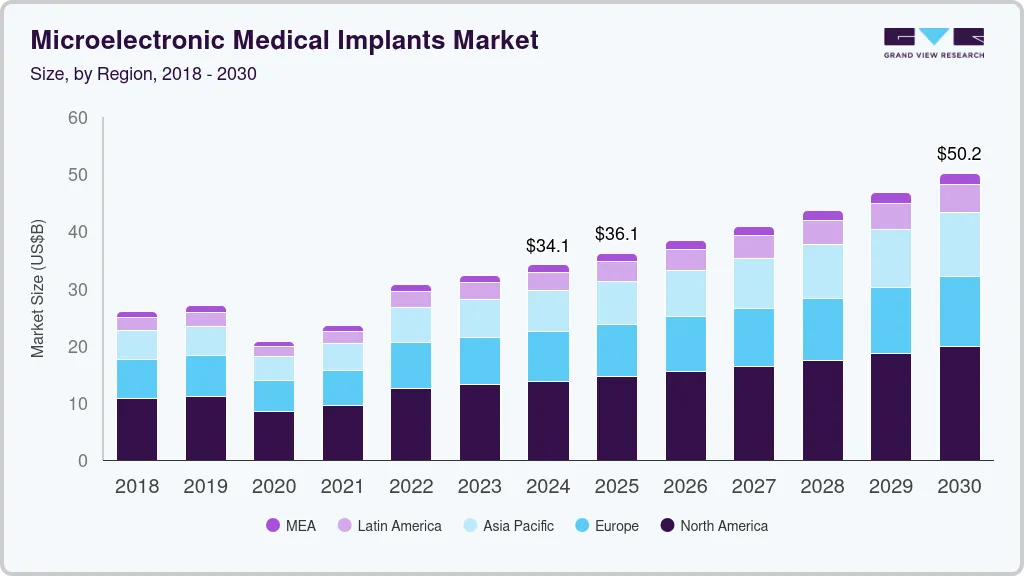
The increasing in geriatric population is leading to increased occurrence of chronic illnesses among the elderly. This is increasing the market for small electronic medical implants used in the management of some age related conditions such as any ailments that are associated with heart failure. The prevalence of heart problems, Parkinson’s disease, and epilepsy is also driving the market growth.
Epilepsy, a neurological disorder wherein a patient experiences epileptic seizures, has also been on a rise, positively impacting demand for microelectronic medical implants. As per WHO, approximately 50 million people have been living with epilepsy, making it one of the most common neurological disorders. Around 80% people with epilepsy live in middle to low-income countries and the risk of premature death in this population is 3 times more than the general population. The disorder can be treated using neurostimulators, which prevent seizures by sending regular and mild pulses of electrical energy to the brain through the vagus nerve. Parkinson’s disease (PD) is a degenerative disorder of the central nervous system, which affects the motor system. As per the study backed Parkinson’s Foundation in 2022, around 90,000 people are diagnosed with PD every year in the U.S.
Product Insights
The pacemakers & defibrillators segment accounted for the largest market share of 34.3% in 2023 owing to increasing incidence of cardiac diseases and rising geriatric population. Common conditions for which a patient may require a pacemaker are heart blockage and bradycardia. It utilizes electrical signals to stimulate the heart muscle to contract and sustain a regular heart rate. It helps in regulating slow heart rate or irregular heartbeats. The emergence of arrhythmias is causing several medical issues, for which these devices are used to assist in controlling the heart to improve health and possibly prevent fatalities.
Neurostimulators is expected to register the fastest CAGR of 7.4% during the forecast period. The rising cases of neurological disorders such as Parkinson’s disease, depression, and epilepsy. The benefit of neurostimulators, for such medical ailments, is that they are less invasive and in the majority of cases effective for such conditions. Moreover, there is an increasing recognition and awareness among the public of neurostimulation therapy and the advantages associated with it.
Technology Insights
The Radiofrequency (RF) technology segment accounted for the largest market share in 2023. RF technology is used in wireless implants to communicate with the circuitry outside the body. It helps researchers and clinicians monitor patient health, capture physiological data, and study normal and abnormal functioning of the body. This RF technology allows doctors to oversee from a distance a patient’s implant data inclusive of vitals and medication. This makes it possible to intervene early and give the best care possible to the patient without the necessity of involving the frequent hospital visits.
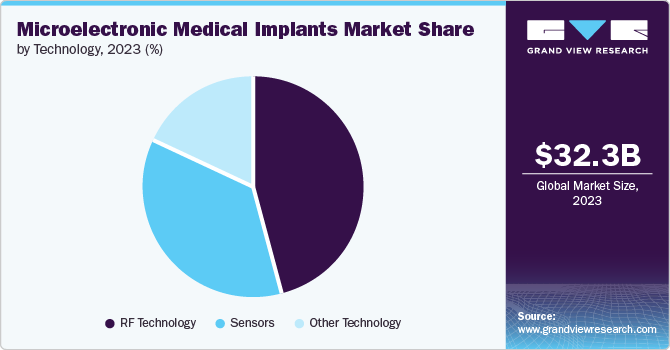
The sensors segment is projected to grow at the fastest CAGR over the forecast period. Sensor enables real-time tracking of different physiological parameters including blood glucose level, muscle movement, pressure, brain activity, and temperature. This help doctors to know the patient’s medical condition more effectively. It helps early detection of any complex medical condition or uncertainties and provides better treatment of chronic diseases, enhancing patients’ results.
Regional Insights
North America microelectronic medical implants market dominated the market in 2023. The widespread use of advanced medical technology is attributing to the market growth in the region. In addition, the presence of medical professionals and hospitals that offers advanced healthcare facilities are expected to propel the market growth.
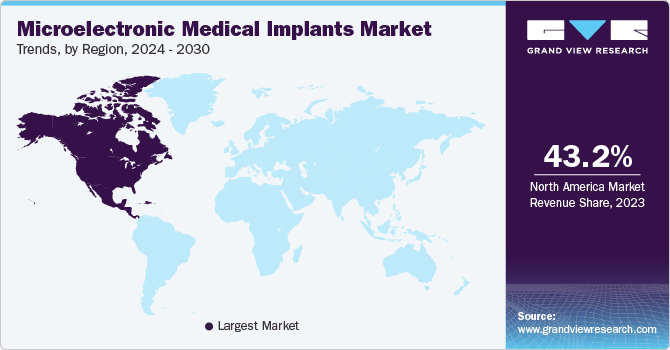
U.S. Microelectronic Medical Implants Market Trends
U.S. microelectronic medical implants market dominated the North America market with a share of 81.4% in 2023 due to technological advancements and government funding. Organizations such as the National Institutes of Health (NIH), provide substantial financial support for the advancement of medical technologies. This encourages innovation and the development of enhanced medical devices.
Europe Microelectronic Medical Implants Market Trends
Europe microelectronic medical implants market identified as a lucrative region in 2023 due to the growing population of elderly citizens, who are at higher risk of getting diagnosed with chronic diseases.
The UK microelectronic medical implants market is expected to grow rapidly in the coming years due to the well-developed healthcare infrastructure, which has skilled professionals and advanced medical equipment. The availability of these professionals who are trained in using implants effectively drives the demand for medical implant market.
Asia Pacific Microelectronic Medical Implants Market Trends
Asia Pacific microelectronic medical implants market anticipated to witness significant growth owing to massive untapped market potential, improving healthcare facilities, and rising geriatric population in this region. In addition, government spending on developing healthcare infrastructure is also growing in this region. This is likely to improve accessibility and affordability of healthcare among several countries, which could benefit the APAC market. Growing medical tourism in the region will also support the growth of this market.
The China microelectronic medical implants market is expected to grow in the coming years. The growing population in China, especially the elderly segment, is generating a significant demand for advanced medical technologies. The rising number of chronic diseases such as cardiovascular disorders, and epilepsy, along with cardiac arrest, heart failure, and others are driving the need for microelectronic medical implants.
Key Microelectronic Medical Implants Company Insights
Some key companies in the microelectronic medical implants market include Zimmer Biomet, Medtronic, Abbott, ZOLL Medical Corporation, LivaNova PLC, Koninklijke Philips N.V., and others.
-
Cochlear Limited offers implantable hearing solutions and sound processors. The company develops, produces, and sells implantable hearing solutions.
Key Microelectronic Medical Implants Companies:
The following are the leading companies in the microelectronic medical implants market. These companies collectively hold the largest market share and dictate industry trends.
- Zimmer Biomet
- Cochlear Ltd.
- Medtronic
- Abbott
- ZOLL Medical Corporation
- Boston Scientific Corporation
- LivaNova PLC
- Biotronik
- Schiller
- Koninklijke Philips N.V.
- Johnson & Johnson Vision
Recent Developments
-
In July 2024, Amvia Sky announced the launch of Amvia Sky HF-T QP pacemaker in Canada. It offers various advanced features, including atrial arrhythmia management tools, CRT AutoAdapt, streamlined care pathways, Next-generation MRI access with 24/7 MRI Guard and 20 left ventricular pacing polarities.
-
In February 2024, Johnson & Johnson MedTech announced the launch of its Tecnis Puresee Intraocular Lens (IOL) in Europe, Middle East, and Africa (EMEA)
-
In November 2022, Cochlear Limited announced that it received FDA approval for Cochlear Nucleus 8 Sound Processor. It features an organization’s innovative technology that automatically adjusts its listening setting based on the changes that occur in person’s environment.
Microelectronic Medical Implants Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 36.14 billion
Revenue forecast in 2030
USD 50.18 billion
Growth Rate
CAGR of 6.6% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, technology, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, South Korea, Australia, Thailand, Brazil, Argentina, Saudi Arabia, Kuwait, UAE, and South Africa
Key companies profiled
Zimmer Biomet; Cochlear Ltd.; Medtronic; Abbott; ZOLL Medical Corporation; Boston Scientific Corporation; LivaNova PLC; Biotronik; Schiller; Koninklijke Philips N.V., • Johnson & Johnson Vision
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Microelectronic Medical Implants Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global microelectronic medical implants market report based on product, technology, and region.
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Pacemakers & Defibrillators
-
Neurostimulators
-
Implantable Drug Pumps
-
Spinal Fusion Stimulators
-
Cochlear Implants
-
Ocular Implants
-
Others
-
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
RF Technology
-
Sensors
-
Other Technology
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
KSA
-
UAE
-
South Africa
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










