- Home
- »
- Biotechnology
- »
-
Next-generation Sequencing Services Market Report, 2030GVR Report cover
![Next-generation Sequencing Services Market Size, Share & Trends Report]()
Next-generation Sequencing Services Market Size, Share & Trends Analysis Report By Service Type (Human Genome Sequencing, Gene Regulation Services), By Workflow, By End-use, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-2-68038-630-1
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Market Size & Trends
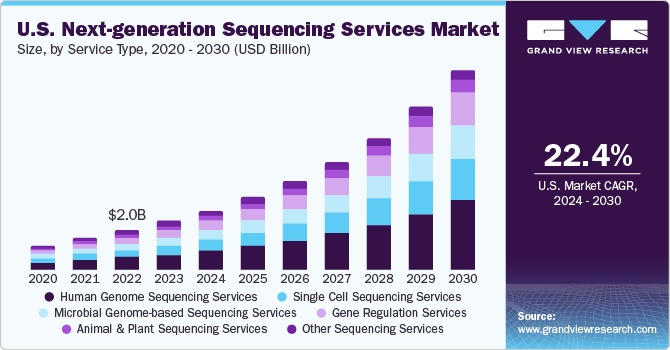
The global next-generation sequencing services market size was estimated at USD 5.89 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 22.68% from 2024 to 2030. Next-generation sequencing (NGS) technology is increasingly leveraged for both conventional and non-conventional applications to gain rapid and comprehensive insights at a genomic level. This is primarily due to continuous improvements in automation, ancillary protocols, services, and analytical solutions in this field. This, in turn, has provided healthy growth prospects for the implementation of NGS in clinical workflows. Such factors are expected to propel industry growth.
The market has been significantly and positively impacted by COVID-19. During the outbreak, assays such as the SARS-CoV-2 assay were utilized to diagnose COVID. As people became more aware of the benefits of next-generation sequencing, the market expanded dramatically. Several firms have acquired FDA approval, paving the path for market's expansion. Several tests have been launched by manufacturers during this pandemic phase, accelerating the NGS services market's growth rate. For example, in March 2022, ARUP Laboratories declared the launch of new coronavirus tests, and IDbyDNA, Inc. collaborated with another firm to introduce new tests for coronavirus, an NGS test for respiratory infections, to assist physicians in screening patients with pneumonia and other respiratory diseases.
The rate of market expansion is expected to be driven by an increase in federal funding. Furthermore, the rise & expansion of the healthcare industry, driven by both private and public players, will generate lucrative market growth prospects, particularly in developing economies. Moreover, increasing utilization of companion diagnostics in order to improve the safety & efficacy of treatments, increased investment in development of innovative healthcare products and devices, along with growing awareness regarding the benefits of multiplex assays all have a positive impact on market growth rate.
Market Dynamics
The decline in sequencing cost, a number of NGS platforms are being developed along with increase in size of sequence databases, which in turn helps target more specific medical conditions. It also enhances the adoption of sequencing technologies across a number of research centers and helps maintain specificity of experiments.
Advancements in genetic testing are expected to drive the adoption of NGS services in clinical laboratories. NGS has potential in diagnosis of rare medical conditions and identification of precision medicine therapies. Implementation of NGS technology and services in clinical laboratories offers access to genetic information, which can be used as prognostic and predictive markers of diseases. Key strategic developments are initiated by key players, healthcare agencies and government investments for implementation of NGS technology and services in clinical diagnosis.
End-use Insights
Based on end-use, the universities & other research entities segment held the largest market share of 53.11% in 2023. Major factors that drive the adoption of NGS services by universities & other research entities include rise in R&D funding, high demand for NGS services, and increasing number of comparative and evolutionary genomic studies in universities. In addition, an increase in the number of R&D activities and projects undertaken by universities and other research entities is expected to create lucrative growth opportunities for NGS service providers in the near future. Recently, in February 2022, The University of the West Indies (The UWI) Mona Campus has established a Next Generation Sequencing (NGS) Service in the Department of Microbiology in Faculty of Medical Sciences, in a partnership with University Hospital of the West Indies (UHWI) along with Ministry of Health and Wellness (MOHW). The NGS service was launched as part of the country's national response to the COVID-19 pandemic.
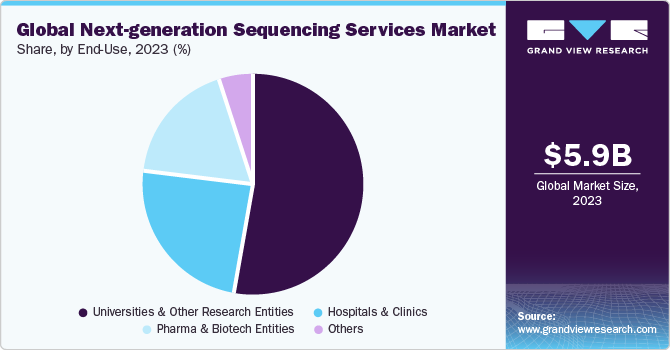
The hospitals & clinics segment is expected to experience the fastest CAGR of 24.03% from 2024 to 2030. This trend in clinical settings is marked by the growing implementation of NGS technology for detection of fetal aneuploidies through analyzing fetal cfDNA obtained from a single maternal blood draw in prenatal diagnostic procedures. Thus, it will increase the demand for NGS services and further boost segment growth.
Service Type Insights
Based on service type, the human genome segment held the largest market share of 30.86% in 2023 and is expected to witness the fastest CAGR of 24.39% from 2024 to 2030. Human genome sequencing services offer access to genomic information related to human genes and deliver solutions for personalized medication, biomarker discovery, information about cancer-associated genes, and pharmacogenomics. For instance, GENEWIZ, Inc. uses sequencing platforms such as Illumina HiSeq X Ten and provides services related to genome sequencing such as detection of germline variants, somatic variation, whole genome re-sequencing, de novo genome assembly, and discovery of structural variants. Hence, propelling the segment growth.
Factors such as low cost of human genome sequencing and rise in adoption of genome mapping projects globally boost market growth. Moreover, the launch of a new NGS device, HiSeq X Ten, allows researchers to access 20,000 genomes available in the Trans-Omics for Precision Medicine (TOPMed) program, sponsored by the National Heart, Lung and Blood Institute (NHLBI). The aforementioned factors have led to advancements in the field of precision medicine and other similar areas that intend to accumulate human genome data. This human genomic data helps provide appropriate human reference genomes for development of personalized medicines.
Workflow Insights
Based on workflow, the sequencing segment held the largest market share of 55.15% in 2023. This high share can be attributed to the fact that companies are undertaking various scientific endeavors to boost the adoption of sequencing services such as receiving certification from Illumina as global service provider of NGS services. These companies are offering a broad array of NGS services for a wide spectrum of sample types. For example, Novogene Corporation has conducted approximately 10,000 projects on numerous samples in 4 years. The company generates publication-ready data for the success of research programs of customers from various domains such as agricultural, biomedical, and environmental science.
NGS services offered in the market include various aspects of sequencing, ranging from consultation to final result derivation. Some companies offer thorough protocol while others are engaged in offering standalone services to customers that have a lab equipped with NGS facilities but seek to outsource some portions of the NGS workflow. This is expected to increase the revenue share of the market.
Regional Insights
North America held the largest market share of 45.87% in 2023,owing to factors such as a well-established informatics network, effective regulatory guidelines pertaining to approval and usage of genetic tests, and the presence of market leaders in the region. In addition, the growing number of FDA-approved genomic tests in the U.S. is anticipated to drive the regional market throughout the forecast period.
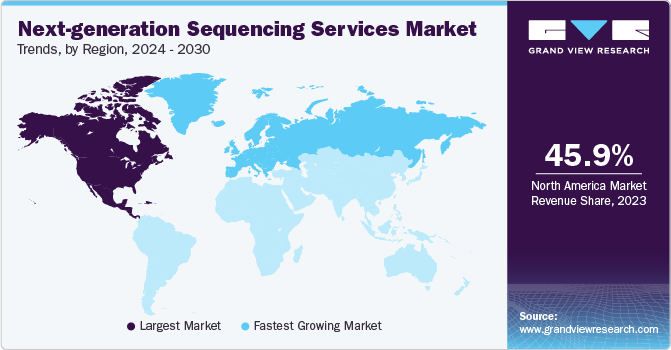
Europe is expected to experience the fastest CAGR of 24.32% from 2024 to 2030. Key factors such as presence of untapped opportunities, economic development, improving healthcare infrastructure, and others are some factors accounting for this rapid growth. Moreover, growing attention of government bodies toward the maintenance of public health and consequent investments in development of advanced diagnostic approaches are anticipated to spur the uptake of seq-services across regions.
Key Companies & Market Share Insights
The continuous demand for next generation sequencing services by multiple end-use has created numerous market opportunities for major players to capitalize on.
-
In July 2023, The University of the Free State's Next Generation Sequencing (UFS-NGS) Unit, part of the Faculty of Health Sciences, recently hosted a successful Data & Bioinformatics Workshop on the UFS's Bloemfontein Campus. The program was aimed to provide participants with the fundamental bioinformatics skills needed to analyze NGS data, with an emphasis on three areas: whole genome data, 16S/ITS metagenomics data, & microbial metagenomics data.
-
In November 2021, Novogene stated during the 44th Annual Gathering of Molecular Biological Society of Japan (MSBJ) in Japan that they will be launching single-cell sequencing services specifically for their Japanese customers. Novogene Japan K.K. will provide on-site service to assist clients in optimizing their single-cell planning with this new service.
-
In November 2021, Illumina and Genetic Alliance announced iHope Genetic Program aspires to provide WGS (whole-genome sequencing) access to thousands of people suffering from genetic illness around the world. Majority of iHope Genetic Health's initiatives will be centered on areas of the globe in need beyond the U.S., with over one-third of Illumina's endorse being devoted to patients in Africa. This will help accelerate start-up operations and upgrade existing operations in terms of sequencing volume and efficiency.
Key Next-Generation Sequencing Services Companies:
- Quest Diagnostics Incorporated
- ARUP Laboratories
- Applied Biological Materials, Inc. (abm)
- Novogene Co, Ltd.
- Azenta Life Sciences (GENEWIZ)
- NanoString
- Illumina, Inc.
- PacBio
- Veritas
- BGI (Beijing Genomics Institute)
- Gene by Gene Ltd.
- Lucigen Corporation
Next-generation Sequencing Services Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 7.18 billion
Revenue forecast in 2030
USD 24.48 billion
Growth rate
CAGR of 22.68% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Report updated
November 2023
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Service type, workflow, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; Switzerland; China; Japan; India; South Korea; Australia; Thailand; Singapore; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Quest Diagnostics Incorporated; ARUP Laboratories; Applied Biological Materials, Inc. (abm); Novogene Co, Ltd.; Azenta Life Sciences (GENEWIZ); NanoString; Illumina, Inc.; PacBio; Veritas; BGI (Beijing Genomics Institute); Gene by Gene Ltd.; Lucigen Corporation
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
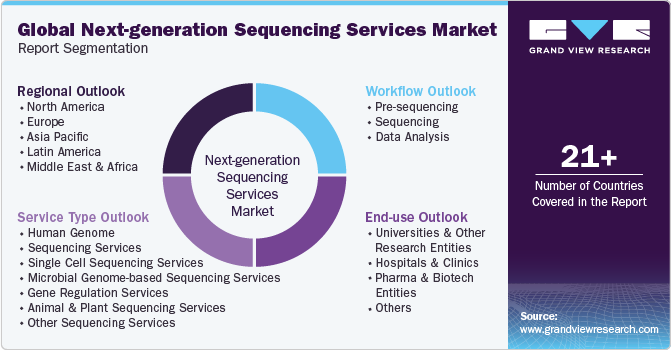
Global Next-generation Sequencing Services Market Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global next-generation sequencing services market report based on service type, workflow, end-use, and region:
-
Service Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Human Genome Sequencing Services
-
Single Cell Sequencing Services
-
Microbial Genome-based Sequencing Services
-
Gene Regulation Services
-
Small RNA Sequencing
-
ChIP Sequencing
-
Other Gene Regulation-based Services
-
-
Animal & Plant Sequencing Services
-
Other Sequencing Services
-
-
Workflow Outlook (Revenue, USD Million, 2018 - 2030)
-
Pre-sequencing
-
Sequencing
-
Data Analysis
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Universities & Other Research Entities
-
Hospitals & Clinics
-
Pharma & Biotech Entities
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Switzerland
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
Singapore
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global next-generation sequencing services market size was estimated at USD 5.89 billion in 2023 and is expected to reach USD 7.18 billion in 2024.
b. The global next-generation sequencing services market is expected to grow at a compound annual growth rate of 22.68% from 2024 to 2030 to reach USD 24.48 billion by 2030.
b. Human genome sequencing services dominated the NGS services market with a share of 30.86% in 2023 owing to high penetration of sequencing in deciphering human genetic profiles.
b. Some key players operating in the NGS services market include Illumina, Inc.; Veritas Genetics; BGI; GENEWIZ Germany GmbH; ABM Inc.; ARUP Laboratories; Novogene Corporation; Lucigen; Quest Diagnostics; and Gene by Gene.
b. Key factors that are driving the next-generation sequencing services market growth include declining sequencing price, increasing implementation of NGS in clinical workflows, and rising market competition between the players to gain substantial revenue share in the market.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





