- Home
- »
- Medical Devices
- »
-
Patient Monitoring Accessories Market Size Report, 2033GVR Report cover
![Patient Monitoring Accessories Market Size, Share & Trends Report]()
Patient Monitoring Accessories Market (2025 - 2033) Size, Share & Trends Analysis Report By Product (SpO2 Sensors, BMS Sensors, BP Cuffs, NMT Sensors, Temperature Sensors, CO2 Absorbers, ECG Leadwires), By Region, And Segment Forecasts
- Report ID: GVR-4-68039-238-0
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2021 - 2023
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Patient Monitoring Accessories Market Summary
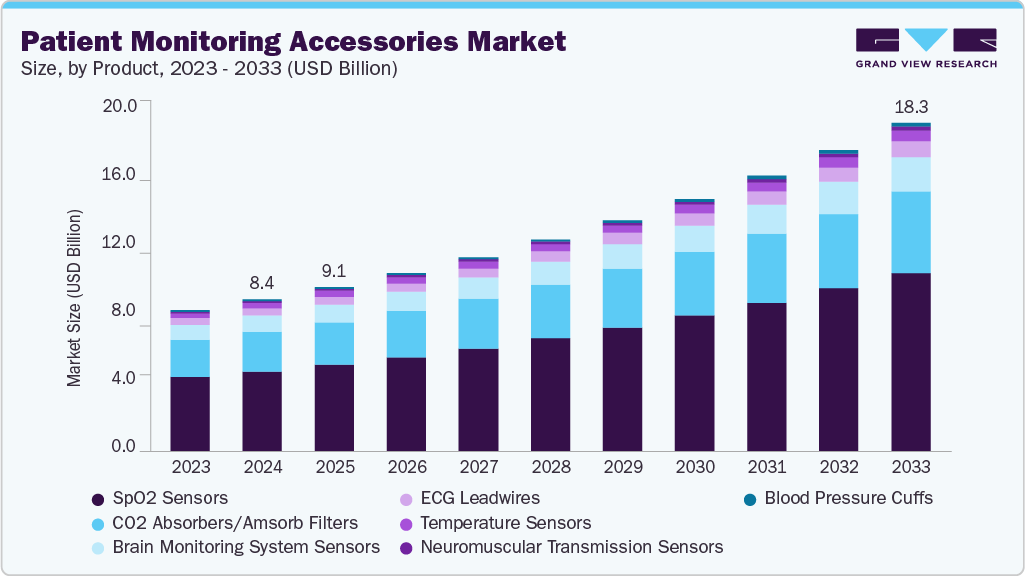
The global patient monitoring accessories market size was estimated at USD 8.44 billion in 2024 and is anticipated to reach USD 18.25 billion by 2033, expanding at a CAGR of 9.05% from 2025 to 2033. Key factors fueling market growth include rapid technological advancements, a rising burden of chronic diseases, an aging global population, and increased adoption of home-based monitoring devices.
Key Market Trends & Insights
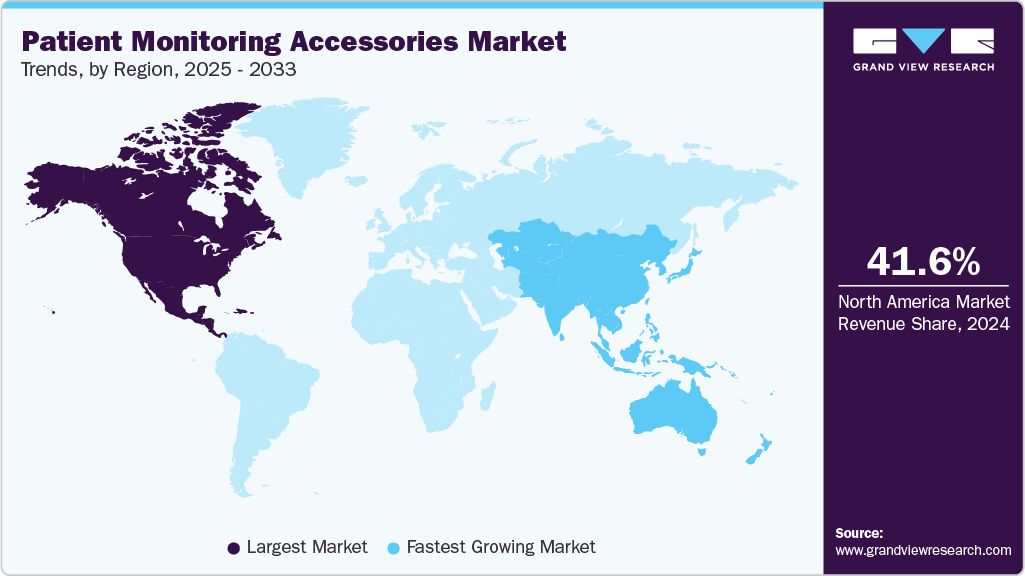
- North America patient monitoring accessories market dominated global market in 2024 and accounted for the largest revenue share of 41.56%.
- U.S. patient monitoring accessories market is anticipated to register the fastest growth rate during the forecast period.
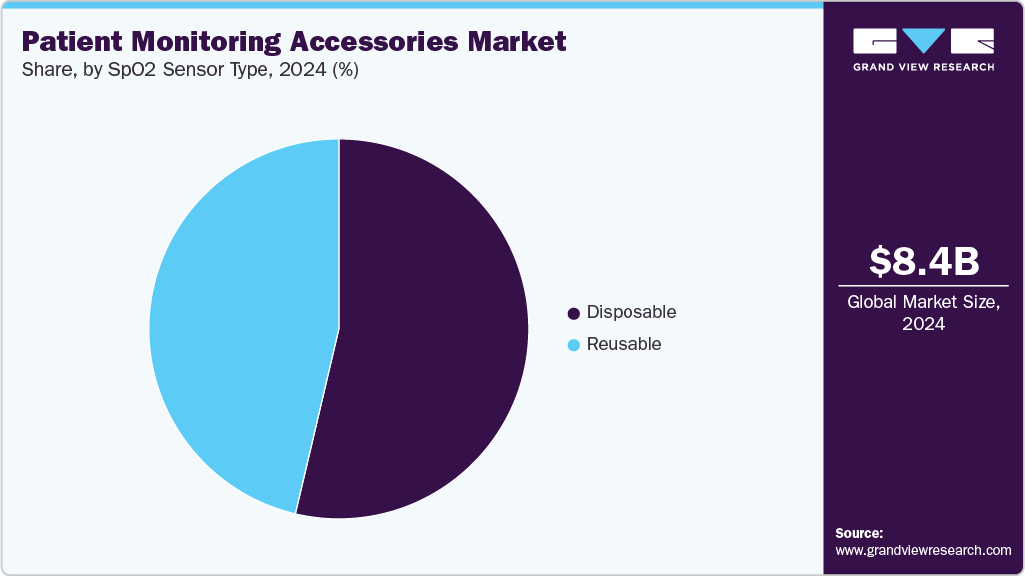
- In terms of product segment, the spO2 sensors segment held the largest revenue share of 52.61% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 8.44 Billion
- 2033 Projected Market Size: USD 18.25 Billion
- CAGR (2025-2033): 9.05%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
The ongoing shift toward preventive care and the need for real-time, continuous patient data are further accelerating demand for advanced monitoring accessories across healthcare settings. The prevalence of chronic diseases such as cardiovascular diseases, diabetes, and chronic respiratory diseases requires frequent assessment of the patient’s health. This creates a demand for accessories that would enable the monitoring of a patient’s health all the time. In addition, there are technological advancements that have ensured the invention of better and more accurate monitoring tools for patients. In October 2024, the CDC highlighted that 60% of U.S. adults suffer from at least one chronic disease, and 40% have two or more. Conditions such as heart disease, cancer, and diabetes remain the top contributors to death, disability, and rising healthcare costs, totaling USD 4.5 trillion annually. These diseases are largely linked to modifiable lifestyle factors like smoking, poor diet, lack of physical activity, and heavy alcohol use.As the geriatric population increases all over the world, there is a requirement of constant health supervision to deal with the illnesses associated with aging. In addition, there is a trend towards the use of home care solutions in families due to the convenience and cost factors. Patient monitoring accessories help people to track their health status from the comfort of their homes, thus ensuring early diagnosis and management of their conditions.In October 2024, the WHO projected that the number of people aged 60 and above will reach 2.1 billion by 2050, with 80% living in low- and middle-income nations. Older adults commonly experience conditions like hearing impairment, osteoarthritis, dementia, diabetes, and chronic respiratory diseases. The prevalence of multiple coexisting health problems in this population is driving the need for more comprehensive, coordinated care solutions.
Healthcare organizations have shifted their focus towards prevention of diseases in order to decrease future healthcare costs and improve health before it gets late for the individual. Monitoring related accessories is crucial in the concept of preventive medicine since it allow for early detection of various health signs.In March 2025, according to Circadian Health, a strategic partnership with Tenovi was launched to expand access to remote monitoring devices for chronic disease management. The collaboration integrates over 40 connected accessories such as blood pressure monitors and glucose meters into Circadian’s virtual care platform, enabling real-time data sharing to support early intervention and reduce hospital admissions.
Market Concentration & Characteristics
The global patient monitoring accessories market shows moderate innovation, driven by advancements in wearable sensors, wireless transmission, and integration with digital platforms. Continuous improvements in form factor, battery life, and real-time analytics are enhancing usability across clinical and home settings. Innovation is also being shaped by AI integration, which supports early anomaly detection. In March 2025, according to News-Medical, innovation in the patient monitoring market is being driven by AI-enabled, wireless, and wearable technologies. Key trends include real-time SpO2 tracking, cloud connectivity, and remote monitoring solutions reshaping post-acute and surgical care.
M&A activity is steady, focused on acquiring telemetry, biosensor, and connectivity technologies to expand remote monitoring capabilities. These deals help companies build end-to-end monitoring systems and strengthen their position in the shift toward decentralized care. Consolidation is also driven by the need to offer bundled solutions with monitors and software platforms. Cross-border acquisitions are emerging as a strategy to enter high-growth regional markets.
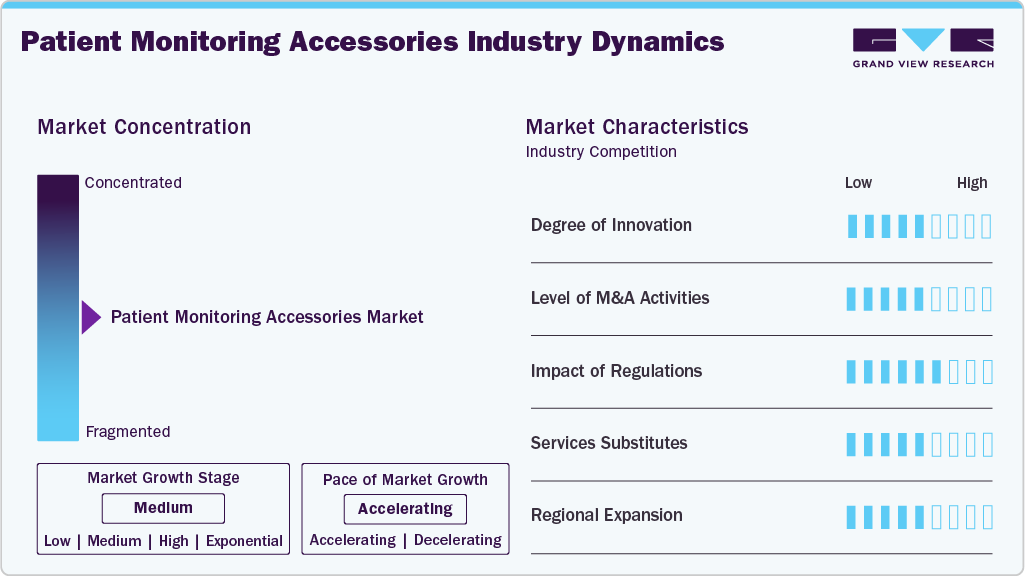
Regulatory oversight is tightening, with requirements from FDA, EU MDR, HIPAA, and GDPR influencing design and deployment. Emphasis is on cybersecurity, interoperability, and real-time data accuracy, especially as accessories become embedded in connected care ecosystems. Regulatory updates increasingly address software-as-a-medical-device (SaMD) elements in accessories. Manufacturers are investing in compliance infrastructure to meet evolving documentation and testing requirements.
Companies are broadening their product lines to serve home care, ambulatory monitoring, and post-acute settings. Efforts include simplifying device interfaces, improving compatibility with existing monitors, and offering scalable solutions for multi-site health systems. Integration with mobile platforms and EHRs is a key area of focus. Some firms are also launching subscription-based models to expand access in outpatient settings.
Regional expansion is moderate to high, with growth concentrated in Asia Pacific, Latin America, and Eastern Europe. Demand is rising due to improved healthcare access and chronic disease burden, though challenges remain around reimbursement models and local training infrastructure. Governments in these regions are increasingly investing in digital health to support remote monitoring. Local partnerships and distributor networks are critical to accelerating adoption and scaling distribution.
Product Insights
The SpO2 sensors segment dominated the market and accounted for a revenue share of 52.61% in 2024 and is expected to retain its dominance over the forecast period. This growth can be attributed to its importance in patient care, diversified use, technological advancements, rising incidence of respiratory diseases, and the growing inclination toward home healthcare and tele monitoring solutions. Advanced types of sensors provide better results, long product life and compatibility with multiple monitoring equipment. In May 2022, Medtronic received FDA clearance for the Nellcor OxySoft SpO₂ sensor, the first pulse oximetry sensor using silicone adhesive to protect neonatal skin while improving adherence and repositionability. The sensor offers enhanced signal acquisition, brighter LEDs, and improved accuracy even in low-perfusion or thick tissue cases, making it suitable for both neonatal and adult critical care patients.
SpO2 sensors are applied in different healthcare facilities such as hospitals, clinics, ambulatory healthcare centers, and home healthcare facilities. Pulse oximetry is another vital monitor to patients which is used to assess the SpO2 levels in the body. This information is crucial during the evaluation of a patient’s respiratory status and general health. In August 2024, Prevounce Health introduced the Pylo OX1-LTE, a cellular-connected SpO₂ sensor for remote patient monitoring. The device delivers accurate oxygen saturation and pulse readings, even in low-signal areas, and is FDA-cleared and FCC-certified. It supports seamless data transmission and is designed for ease of use and high patient adherence.
The Neuromuscular Transmission Sensors (NMT Sensors) segment is expected to grow at the fastest CAGR during the forecast period. This growth can be attributed to the use of technology, the rising incidence of diseases, the shift to patient-centric care and integration with digital health systems. In October 2023, Senzime launched TetraSensitive, the first EMG-based disposable neuromuscular sensor for patients with fragile skin. Made with ultrasoft, stretchable materials, it reduces skin damage while maintaining strong signal quality and works with the TetraGraph monitor.
There is a rising need for better and more accurate monitoring systems for patients with neuromuscular transmission disorders. These sensors work to measure muscle activity, nerve impulse transmission, and blockade of nerve impulses that occur during surgical procedures and other times of intensive care. The sensors of neuromuscular transmission are being incorporated into digital health solutions as well as electronic medical records systems, allowing efficient data capture, analysis and sharing among healthcare providers. For instance, ToFscan by Dräger is a neuromuscular monitoring device that helps assess muscle relaxation in anesthetized patients. It features a 3D accelerometer for accurate thumb movement measurement, is low-maintenance, and supports reliable treatment adjustments.
Regional Insights
North America patient monitoring accessories market held a revenue share of 41.56% in 2024. It is attributed to increasing chronic diseases, technological advancements, well-developed healthcare facilities, availability of healthcare professionals and increasing investment in the healthcare sector by different organizations. In April 2025, according to the CDC’s Behavioral Risk Factor Surveillance System data, 76.4% of US adults (194 million) had at least one chronic condition. Prevalence among young adults (18 to 34) rose from 52.5% in 2013 to 59.5% in 2023. Chronic illness is growing across all age groups, underscoring the need for earlier interventions.
U.S. Patient Monitoring Accessories Market Trends
The patient monitoring accessories market in the U.S. dominated the North America market in 2024 due to the well-established healthcare infrastructure and increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders and respiratory conditions. In October 2023, Ricoh USA, Inc. announced the launch of managed services offering, RICOH Remote Patient Monitoring (RPM) Enablement, which is designed to assist in sustainable & efficient Remote Patient Monitoring (RPM) workflows, thereby improving care delivery team and patient experience.
Europe Patient Monitoring Accessories Market Trends
Europe patient monitoring accessories market was identified as a lucrative region in 2024 due to increasing chronic diseases and early detection. Increasing investment in healthcare infrastructure and technology development is driving the market growth. In October 2024, according to Mindray UK, the company supported new strategic priorities introduced by AXREM to align patient monitoring technologies with NHS goals. The initiative emphasizes patient safety, early diagnosis, and digital connectivity to enhance care delivery across NHS facilities.
The UK patient monitoring accessories market is expected to grow significantly in the coming years due to a rise in healthcare awareness and growing emphasis on preventive healthcare measures aimed at early detection and intervention to prevent serious health complications.In October 2024, according to the Health Foundation, chronic pain cases in England are projected to rise by nearly 2 million by 2040, largely due to ageing and musculoskeletal issues. This underscores the need for early detection tools such as patient monitoring accessories to manage chronic conditions before they escalate.
The Germany patient monitoring accessories market held a substantial market share in 2024 owing to increasing investment in the healthcare segment and technology. Continuous product innovation and increasing competitiveness are expected to drive the market growth. In October 2024, a study published in the Journal of Medical Internet Research spotlighted Germany’s move to integrate data from wearable patient monitoring accessories into electronic health records. This shift allows continuous remote tracking of vitals such as heart rate and activity levels, reinforcing the clinical utility of accessories like smartwatches and sensor patches in patient monitoring.
Asia Pacific Patient Monitoring Accessories Market Trends
Asia Pacific patient monitoring accessories market is anticipated to witness the fastest CAGR in the coming years. This growth is owing to the significantly increasing investments in healthcare facilities. In June 2024, Israel’s Medasense partnered with Nihon Kohden for the distribution of its pain monitoring accessory, the PMD-200, in Japan. The AI-powered device uses a non-invasive sensor to quantify pain levels in real time, aiding personalized anesthesia.This marks a step forward in integrating smart accessories into surgical and critical care monitoring.
Patient monitoring accessories market in India is expected to grow rapidly from 2025 to 2033 due to the rising prevalence of chronic diseases due to lifestyle changes, growing preference for remote and home monitoring and portability of devices. In March 2024, Mindray India was honored at the Precision Med India Awards for its M-Connect IT platform, recognized as the top integration solution for patient monitoring and life support devices. The system streamlines the connection of monitoring accessories with hospital networks, improving clinical efficiency, patient safety, and precision-driven care.
The Japan patient monitoring accessories market is expected to grow rapidly in the coming years due to the rise in healthcare awareness and the increasing geriatric population which requires extensive healthcare and continuous monitoring. In July 2025, iRhythm Technologies launched its Zio® ECG patch in Japan, expanding access to wearable cardiac monitoring accessories in Asia. The single-use patch provides up to 14 days of continuous ECG data, paired with AI-powered software for remote arrhythmia detection.
Latin America Patient Monitoring Accessories Market Trends
Latin America's patient monitoring accessories market is growing steadily, supported by rising investment in hospital infrastructure, greater emphasis on chronic disease management, and expanding adoption of remote monitoring. Improvements in healthcare delivery and broader digital health initiatives are also accelerating market development. In July 2025, Brazil’s UniSALESIANO invested in tablets for community health agents in Araçatuba to improve home-based care and real-time patient monitoring. These accessories support offline data entry, geolocation, and clinical tracking modernizing primary care services in underserved areas.
Brazil’s patient monitoring accessories market is expanding due to its large aging population and increasing demand for continuous monitoring in both acute and outpatient settings, particularly for cardiovascular and respiratory conditions.In June 2025, Dong-A ST launched HiCardi+ in Brazil, a remote monitoring accessory that tracks ECG, heart rate, skin temperature, and respiration. The system includes a wearable patch and software that allows clinicians to access patient data remotely. This reflects rising demand for advanced patient monitoring accessories in Latin America.
MEA Patient Monitoring Accessories Market Trends
The Middle East and Africa patient monitoring accessories market are experiencing rising demand driven by an increase in lifestyle-related chronic diseases and the push toward digital health modernization across public and private sectors.In March 2025, according to Scientific Reports, over half of adults aged 40+ in rural South Africa were found to have multiple chronic conditions, including diabetes, HIV, and hypertension. The study highlights a growing burden of multimorbidity and the urgent need for integrated chronic disease care.
Saudi Arabia patient monitoring accessories market is expected to grow at a strong CAGR due to rising investments in hospital digitization, broader deployment of wearable monitors, and government-led initiatives focused on early disease detection and home-based care.In February 2023, Chronolife partnered with SAMI Advanced Electronics in Saudi Arabia to co-develop wearable health monitoring devices for high-risk industries such as defense and energy. The initiative aims to enhance worker safety through real-time physiological tracking, aligning with Saudi Arabia’s Vision 2030 focus on healthcare innovation and localized tech development.
Key Patient Monitoring Accessories Company Insights
Some of the key companies in the patient monitoring accessories market include Welch Allyn Warehouse; LifeScan IP Holdings, LLC; Honeywell International Inc.; Koninklijke Philips N.V.; OMRON Corporation and F. Hoffmann-La Roche Ltd. Organizations in the market are focusing on increasing customer base to gain a competitive edge in the industry. Therefore, key players are taking several strategic initiatives, such as mergers and acquisitions, and partnerships with other major companies.
-
Honeywell International Inc. is a provider of a range of patient monitoring accessories. Honeywell’s offerings include SpO2 sensors, ECG leads, and other essential components used in patient monitoring systems.
Key Patient Monitoring Accessories Companies:
The following are the leading companies in the patient monitoring accessories market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic
- GE HealthCare
- NIHON KOHDEN CORPORATION
- Masimo
- Welch Allyn Warehouse
- LifeScan IP Holdings, LLC.
- Honeywell International Inc.
- Koninklijke Philips N.V.
- OMRON Healthcare Co., Ltd
- F. Hoffmann-La Roche Ltd
Recent Developments
-
In April 2025, OSI Systems’ Spacelabs Healthcare secured a USD 4 million agreement with a U.S. hospital to deliver patient monitoring systems, supplies, and accessories. The deal supports infrastructure modernization and long-term clinical partnerships.
-
In October 2024, OSI Systems’ Spacelabs Healthcare received a USD 6 million order from a U.S. hospital system for patient monitoring solutions and related accessories. The deal includes bedside and portable monitors, central stations, and clinical information systems. This supports expanded deployment of patient monitoring accessories within hospital workflows.
-
In June 2024, Masimo partnered with Cleveland Clinic to launch hospital-based remote patient monitoring. The collaboration integrates Masimo’s Hospital Automation platform with Cleveland Clinic’s central patient monitoring platforms.
Patient Monitoring Accessories Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 9.13 billion
Revenue forecast in 2033
USD 18.25 billion
Growth rate
CAGR of 9.05% from 2025 to 2033
Base year for estimation
2024
Historical data
2021 - 2023
Forecast period
2025 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Thailand; Brazil; Argentina; Saudi Arabia; UAE; Kuwait; South Africa
Key companies profiled
Medtronic; GE HealthCare; NIHON KOHDEN CORPORATION; Masimo; Welch Allyn Warehouse; LifeScan IP Holdings, LLC; Honeywell International Inc.; Koninklijke Philips N.V.; OMRON Corporation and F. Hoffmann-La Roche Ltd
Customization scope
Free report customization (equivalent up to 8 analysts' working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Patient Monitoring Accessories Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the global patient monitoring accessories market report based on product and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
SpO2 Sensors
-
Disposable
-
Reusable
-
-
Blood Pressure Cuffs
-
Disposable
-
Reusable
-
-
Brain Monitoring System Sensors
-
Neuromuscular Transmission Sensors
-
Temperature Sensors
-
Adult
-
Pediatric
-
-
CO2 Absorbers/Amsorb Filters
-
ECG Leadwires
-
Disposable
-
Reusable
-
-
-
Regional Outlook (Revenue, USD Million; 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global patient monitoring accessories market size in 2024 was estimated at USD 8.44 billion and is expected to reach USD 9.13 billion in 2025.
b. The global patient monitoring accessories market is expected to grow at a compound annual growth rate of 9.05% from 2025 to 2033 to reach USD 18.25 billion by 2033.
b. The North American region dominated the global market with a share of 41.56% in 2024. This can be attributed to increasing chronic diseases that need routine monitoring coupled with the presence of sophisticated healthcare infrastructure.
b. Some key players operating in the global patient monitoring accessories market include Medtronic plc, GE Healthcare Limited, Nihon Kohden Corporation, Masimo, Welch Allyn Inc. Philips Healthcare, Lifescan Inc, Honeywell, Lifecare Solutions, Phillips, Omron, and Roche.
b. Key factors that are driving the patient monitoring accessories market growth include the high burden and prevalence of chronic illness, rising demand for round the clock monitoring, and the advent of technologically advanced monitoring products.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










