- Home
- »
- Biotechnology
- »
-
Recombinant Proteins Manufacturing Services Market Report 2030GVR Report cover
![Recombinant Proteins Manufacturing Services Market Size, Share & Trends Report]()
Recombinant Proteins Manufacturing Services Market (2023 - 2030) Size, Share & Trends Analysis Report, By Service Type (Pre-clinical & Clinical Services, Commercial Production), By Host Cell, By End-user, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-108-7
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Report Overview
The global recombinant proteins manufacturing services market size was estimated at USD 3.48 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 17.79% from 2023 to 2030. The increasing adoption of outsourcing for recombinant protein manufacturing, the growing preference for biologics & biosimilars, high prevalence of chronic disorders, and increasing applications of proteins are expected to boost market expansion. According to the American Cancer Society, in the U.S., there were an estimated 609,360 cancer deaths and 1.9 million newly diagnosed cancer cases in 2022. As recombinant proteins are widely used in research and diagnosis of cancer, an upsurge in the cancer burden is projected to boost the demand for proteins, driving market growth. Moreover, the high burden of cancer creates a need for advanced therapeutics, leading to increased adoption of outsourcing services for the production of advanced therapies.
Protein production is a cost-intensive process that requires specialized facilities and large-scale production capabilities. Several challenges concerning regulatory compliance and adherence to manufacturing standards can further increase the complexities associated with the production of recombinant protein-based products. As a result, in recent years, the trend toward outsourcing recombinant protein production to leverage the expertise and capabilities of CMOs in this domain has been increasing. Furthermore, certain specialized and repetitive activities, such as fill/finish, analytical testing/bioassays, and toxicology testing, are outsourced to a greater extent in the biopharmaceutical industry.
Recombinant proteins are used in the development of innovative therapeutics for a variety of chronic conditions, including cancer and other rare disorders. The creation of recombinant pharmaceutical proteins in heterologous systems-introducing complementary RNA (cRNA) as well as DNA (cDNA) from one species into cells of another species to produce the desired protein-has seen an increase in demand in recent years. Furthermore, there is an increasing need for advanced protein production platforms since many recombinant protein applications require complex proteins & glycoproteins that are challenging for biotech companies to manufacture.
In addition, with the ongoing advancements in recombinant technology, novel applications of proteins for developing personalized medicine, cell & gene therapies, and bioengineering products are projected to increase. For instance, gene editing technologies, such as CRISPR-Cas9, have enabled the precise alteration of recombinant proteins to create innovative therapeutics. These factors are expected to significantly increase the demand for recombinant proteins, driving the market for manufacturing services.
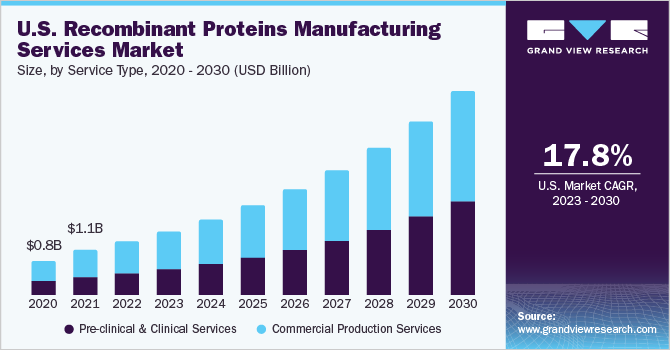
Service Type Insights
The commercial production services segment held the largest market share of 58.60% in 2022. The development of recombinant DNA technology, cell culture methods, protein expression systems, and growing accessibility of high-yield expression systems like mammalian cell lines, yeast, and bacteria, which can boost production productivity & efficiency, are some of the factors driving the commercial production services market.
The pre-clinical & clinical services segment is expected to witness the fastest CAGR of 19.51% from 2023 to 2030. The segment is expected to witness lucrative growth over the forecast period due to an increasing focus on precision medicine, targeted therapies, and the growing need for outsourcing clinical trial design, data collection, and analysis.
Host Cell Insights
The mammalian segment accounted for the largest market share of 54.91% in 2022. For manufacturing complex recombinant proteins that need significant folding, subunit assembly, and/or posttranslational modifications, mammalian cells are appropriate host cells. These features contribute to understanding the preference for mammalian cells utilized in the biotech and pharmaceutical sectors for manufacturing diagnostic and therapeutic proteins.
On the other hand, the yeast & fungi segment is estimated to witness the fastest CAGR of 19.40% from 2023 to 2030. Yeasts are now used in several healthcare fields, including the generation of therapeutic recombinant proteins and their traditional application in fermentation. Yeast cells are particularly appealing as hosts for biopharmaceutical synthesis as they are generally accepted as safe organisms. This is expected to drive market expansion throughout the forecast period.
End-user Insights
The pharmaceutical & biotechnology companies segment held the largest market share of 78.53% in 2022. Numerous major and small biotechnology businesses are driving innovation in terms of service and manufacturing capabilities and are providing commercial potential for market expansion. For instance, in June 2023, Waters and Sartorius strengthened their partnership to provide thorough bioanalytics for downstream biomanufacturing. Furthermore, rising demand for protein manufacturing services, growing competition among players, and a variety of applications by end-users are contributing to the development of the global market.
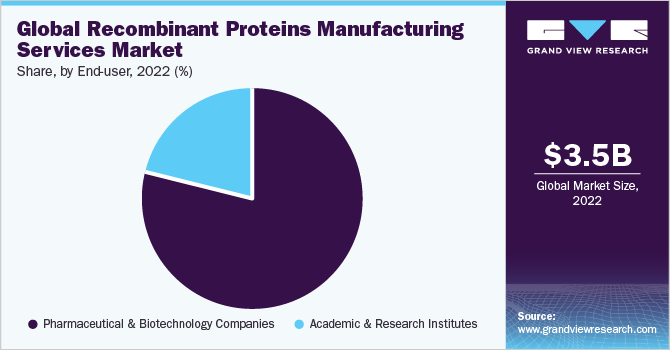
On the other hand, the academic & research institutes industry segment is estimated to witness the fastest CAGR of 19.62% from 2023 to 2030. As recombinant technology advances, novel uses of recombinant proteins enabling the development of customized medicine, cell and gene therapies, and bioengineering products are expected to grow at a rapid rate. For example, the development of gene editing technologies such as CRISPR-Cas9 permitted precise modification of recombinant proteins to develop novel therapies. These factors are projected to significantly improve the demand for recombinant proteins in academic and research institutes, driving the manufacturing services industry.
Regional Insights
North America held the largest market share of 37.86% of the recombinant proteins manufacturing services market in 2022. This can be attributed to increased research spending, the availability of well-established healthcare infrastructure, and the existence of several industry participants. These factors are expected to increase the demand for recombinant protein manufacturing services.
Additionally, it is anticipated that the market will expand due to the rising incidence of chronic disorders in the region. For instance, approximately 25% of persons in the U.S. have two or more chronic illnesses, according to research published in the International Journal of Environmental Research & Public Health (IJEPH) in September 2022. Such a high rate of disease is anticipated to increase the need for recombinant proteins, fueling the market's expansion throughout the forecast period.
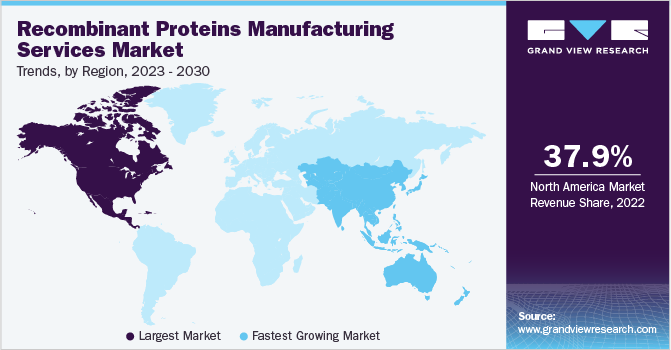
Asia Pacific is expected to experience the fastest CAGR of 21.44% from 2023 to 2030 due to improved healthcare infrastructure, improving economic factors, an increase in government efforts to raise awareness, as well as favorable regulatory policies that will boost the rate of adoption for recombinant manufacturing services. Furthermore, the region's increased need for high-quality healthcare has a favorable impact on the industry.
Key Companies & Market Share Insights
The continuous demand for recombinant protein manufacturing services by multiple applications has created numerous market opportunities for major players to capitalize on. For instance, in April 2023, the LFB Group stated that its subsidiary LFB Biomanufacturing has joined the France BioLead group, which promotes biopharmaceutical manufacturing in France. Some prominent players in the global recombinant proteins manufacturing services market include:
-
Lonza
-
Boehringer Ingelheim International GmbH
-
FUJIFILM Diosynth Biotechnologies
-
Merck KGaA
-
Bruker (InVivo BioTech Services GmbH)
-
Sino Biological, Inc.
-
GenScript
-
Kaneka Corporation (Kaneka Eurogentec S.A)
-
Polyplus Transfection (Xpress Biologics)
-
Boster Biological Technology
-
Trenzyme GmbH
Recombinant Proteins Manufacturing Services Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 4.05 billion
Revenue forecast in 2030
USD 12.74 billion
Growth rate
CAGR of 17.79% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Service type, host cell, end-user, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; Switzerland; Netherlands; China; Japan; India; South Korea; Australia; Thailand; Brazil; Mexico, Argentina; South Africa; Saudi Arabia, UAE; Kuwait
Key companies profiled
Lonza; Boehringer Ingelheim International GmbH; FUJIFILM Diosynth Biotechnologies; Merck KGaA; Bruker (InVivo BioTech Services GmbH); Sino Biological, Inc.; GenScript, Kaneka Corporation (Kaneka Eurogentec S.A); Polyplus Transfection (Xpress Biologics); Boster Biological Technology; Trenzyme GmbH
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
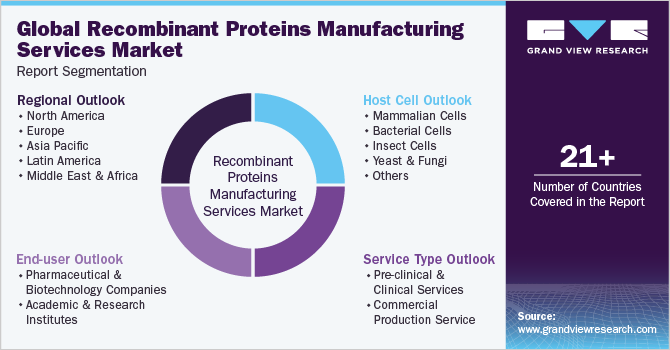
Global Recombinant Proteins Manufacturing Services Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, grand view research has segmented the global recombinant proteins manufacturing services market report based on service type, host cell, end-user, and region:
-
Service Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Pre-clinical & Clinical Services
-
Commercial Production Services
-
-
Host Cell Outlook (Revenue, USD Million, 2018 - 2030)
-
Mammalian Cells
-
Bacterial Cells
-
Insect Cells
-
Yeast & Fungi
-
Others
-
-
End-user Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharmaceutical & Biotechnology Companies
-
Academic & Research Institutes
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Switzerland
-
Netherlands
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global recombinant proteins manufacturing services market size was estimated at USD 3.48 billion in 2022 and is expected to reach USD 4.05 billion in 2023.
b. The global recombinant proteins manufacturing services market is expected to grow at a compound annual growth rate of 17.79% from 2023 to 2030 to reach USD 12.74 billion by 2030.
b. North America dominated the recombinant proteins manufacturing services market with a share of 37.86% in 2022. This is attributable to the presence of several key players in the region and the rising demand for recombinant protein-based therapeutic products.
b. Some key players operating in the recombinant proteins manufacturing services market include Lonza, Boehringer Ingelheim International GmbH, FUJIFILM Diosynth Biotechnologies, Merck KGaA, Bruker (InVivo BioTech Services GmbH), Sino Biological, Inc., GenScript, Kaneka Corporation (Kaneka Eurogentec S.A), Polyplus Transfection (Xpress Biologics), Boster Biological Technology, Trenzyme GmbH.
b. Key factors that are driving the recombinant proteins manufacturing services market growth include the increasing adoption of outsourcing for recombinant protein manufacturing, the growing preference for biologics & biosimilars, the high prevalence of chronic disorders, and the increasing demand for protein-based therapies.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










