- Home
- »
- Medical Devices
- »
-
U.S. Pharmaceutical Dissolution Testing Services Market, 2033GVR Report cover
![U.S. Pharmaceutical Dissolution Testing Services Market Size, Share & Trends Report]()
U.S. Pharmaceutical Dissolution Testing Services Market (2025 - 2033 ) Size, Share & Trends Analysis Report By Method (In Vitro, In Vivo), By Dosage Form (Capsules, Tablets), By Dissolution Apparatus (Basket, Paddle), By Region, And Segment Forecasts
- Report ID: GVR-4-68040-726-7
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
The U.S. pharmaceutical dissolution testing services market size was estimated at USD 224.36 million in 2024 and is projected to reach USD 423.71 million by 2033, growing at a CAGR of 7.41% from 2025 to 2033.Rising demand for generic drugs and bioequivalence studies, constant regulatory emphasis on consistent drug release profiles and quality control, and growing outsourcing activities are driving the market growth.

The U.S. pharmaceutical dissolution testing services industry’s growth is driven by the increasing regulatory stringency surrounding drug quality, safety, and bioavailability. Health authorities such as the U.S. FDA, EMA, and CDSCO mandate dissolution testing as a core requirement in the approval process for both branded and generic pharmaceuticals. This has led to a growing need for advanced, reliable, and standardized dissolution testing to ensure consistent drug release profiles. The rising volume of generic drug approvals globally has intensified the demand for comparative dissolution testing in bioequivalence studies, making it essential for companies to outsource these services for faster and cost-effective regulatory compliance. Moreover, the complexity of modern drug formulations, such as sustained-release and poorly soluble drugs, necessitates customized and high-precision testing protocols, prompting pharma firms to rely on specialized contract service providers with technical expertise and instrumentation capabilities.
Furthermore, the rapid expansion of pharmaceutical R&D pipelines, especially in emerging markets, is creating heightened demand for dissolution testing across early- and late-phase development. Small and mid-sized pharmaceutical and biotech firms, often operating with limited in-house infrastructure, are increasingly turning to third-party service providers for dissolution testing to accelerate timelines and reduce operational burdens. Furthermore, the rise in personalized medicine and novel drug delivery systems, including nanotechnology and 3D-printed tablets, is reshaping dissolution testing needs.Advances in automation, real-time analytics, and integration of dissolution testing with other quality control technologies are also streamlining workflows and improving data accuracy, further incentivizing outsourcing.
Opportunity Analysis
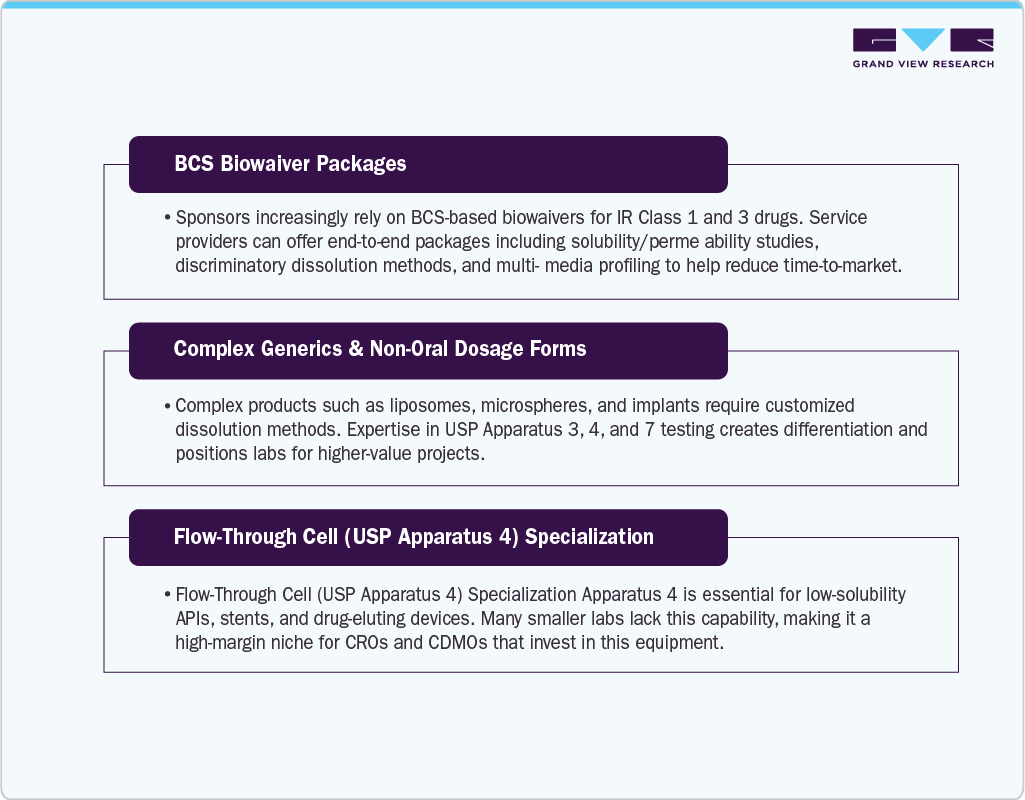
The U.S. pharmaceutical dissolution testing services market is poised for significant growth as drug development becomes more globalized and complex. A major opportunity lies in the increasing demand for dissolution testing in developing in the U.S., where local regulatory bodies are aligning with global standards for drug quality and bioavailability.
Technological Advancements
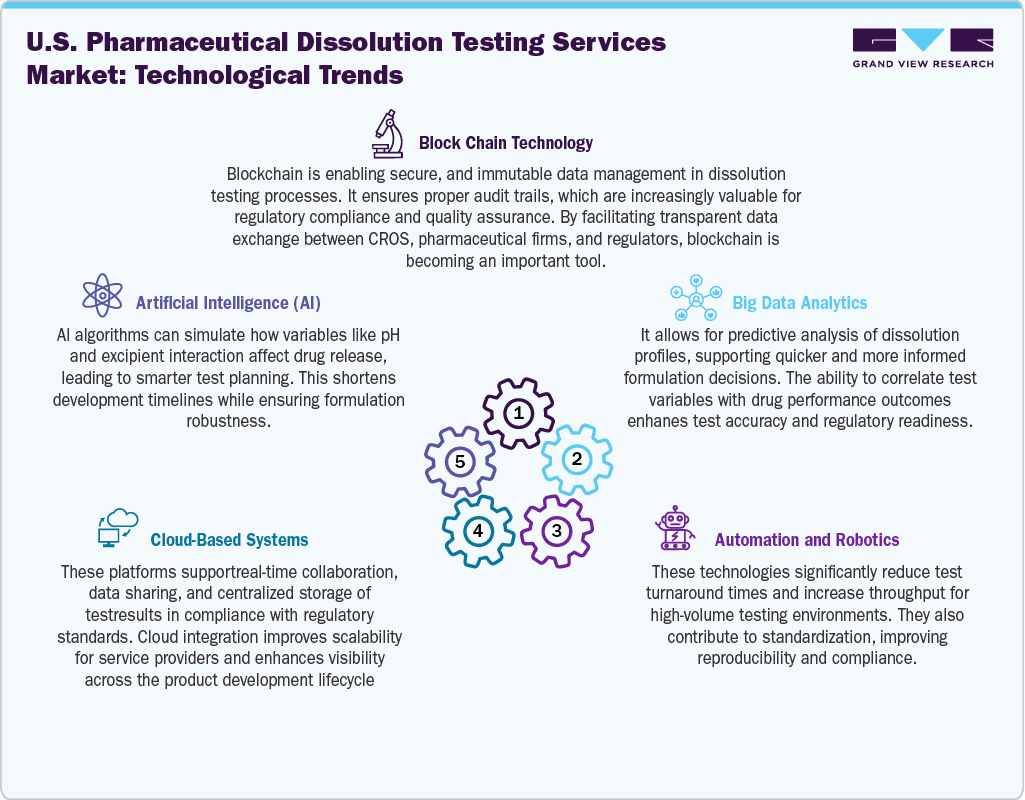
The U.S. pharmaceutical dissolution testing services industry is experiencing significant evolution driven by rapid technological advancements that enhance testing accuracy, efficiency, and regulatory compliance. Automated dissolution systems equipped with robotics and integrated data analytics are reducing manual errors and increasing throughput, especially for high-volume generic drug production. Technologies such as UV fiber optic systems allow real-time monitoring without interrupting the dissolution process, aligning with the principles of Process Analytical Technology (PAT). In addition, the integration of software platforms for 21 CFR Part 11 compliance and cloud-based data management has streamlined regulatory submissions and remote auditing. These innovations not only accelerate formulation development and bioequivalence testing but also expand the service scope of CROs, making advanced dissolution testing more accessible for virtual pharma companies and complex drug developers.
Pricing Analysis
The pricing analysis model for the U.S. pharmaceutical dissolution testing services market is primarily structured around factors such as test complexity, drug type (immediate vs. controlled release), method validation needs, regulatory requirements, and sample volume. Service providers offer tiered pricing models, with basic in vitro dissolution tests priced lower than customized, USP-compliant, or IVIVC-based studies. Pricing also varies based on whether the service includes method development, stability testing, or bioequivalence support. In addition, bundled pricing for high-throughput screening or long-term contracts is increasingly adopted to attract virtual pharma and generic drug developers. At the same time, premium charges apply for rapid turnaround and 21 CFR Part 11-compliant digital reporting.

Method Insights
The in vitro segment accounted for the largest revenue share in the U.S. pharmaceutical dissolution testing services industry with 58.09% in 2024. The growth of the segment is driven by its cost-effectiveness, regulatory acceptance, and widespread use in early-stage drug development and quality control. In vitro methods are less complex, quicker to execute, and do not require human or animal subjects, making them ideal for high-throughput screening of generic formulations and routine batch testing.
The in vivo segment is anticipated to grow at a lucrative CAGR during the forecast period. The segment growth is driven due to its crucial role in bridging in vitro data with real-world drug performance. The growing need for IVIVC (in vitro-in vivo correlation) studies, especially for complex formulations like controlled-release and poorly soluble drugs, is fueling this demand. In addition, innovations in pharmacokinetic modeling and increasing regulatory support for biorelevant dissolution assessments are making in vivo testing services more strategic and necessary.
Dosage Form Insights
The tablets segment held the largest share of the U.S. pharmaceutical dissolution testing services market in 2024.This growth can be attributed to the widespread use of solid oral dosage forms in both branded and generic pharmaceuticals. Tablets are the most common and cost-effective drug delivery method, and regulatory agencies mandate robust dissolution testing for quality assurance, bioequivalence, and batch consistency. This segment benefits from a high volume of generic product filings and routine quality control testing.
The capsule segment is anticipated to grow at the second-fastest CAGR over the forecast period. The growth of the segment is due to its increasing application in specialty drugs and modified-release formulations. The rising demand for personalized therapies, along with innovations in capsule-based multiparticulate systems, is pushing the need for more sophisticated and tailored dissolution testing protocols, particularly in bio-relevant media.
Dissolution Apparatus Insights
The paddle segment held the largest share of the U.S. pharmaceutical dissolution testing services market in 2024 due to its widespread use and versatility in testing various dosage forms. Its adoption is due to the paddle apparatus's ability to provide consistent and reliable dissolution results, making it a preferred choice among pharmaceutical companies.
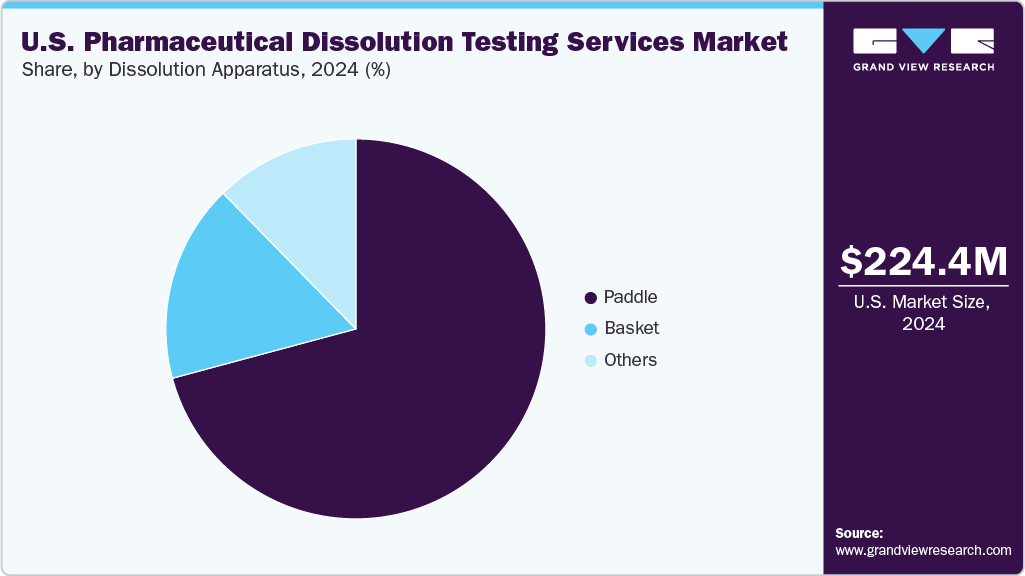
In addition, the basket segment is anticipated to witness the second-fastest CAGR growth during the forecast period. This growth can be attributed to its suitability for testing formulations that tend to float or have low density, which the paddle method may not handle as effectively. Increasing demand for specialized dissolution testing methods to accommodate diverse drug formulations is driving the basket segment's expansion, positioning it as a key growth area within the overall dissolution testing services market.
Key U.S. Pharmaceutical Dissolution Testing Services Company Insights
Several key players are acquiring various strategic initiatives to strengthen their market position, offering diverse services to customers. The prominent strategies adopted by companies are service launches, mergers & acquisitions/joint ventures, mergers, partnerships & agreements, expansions, and others to increase market presence & revenue and gain a competitive edge drives the market growth.
Key U.S. Pharmaceutical Dissolution Testing Services Companies:
- Intertek Group Plc.
- Avivia BV
- Almac Group
- Agilent Technologies, Inc.
- Catalent, Inc.
- Thermo Fisher Scientific Inc.
- Cambrex
- Charles River Laboratories
- Boston Analytical
- Pace Analytical Life Sciences
- SOTAX
- AMRI
- SGS SA
Recent Developments
- In February 2025, Thermo Fisher Scientific Inc. acquired the Solventum's Purification & Filtration business for USD 4.1 billion. The acquisition aims to strengthen the company’s ability to offer integrated, end-to-end drug development solutions, increasing demand from pharmaceutical companies for comprehensive testing and purification services.
U.S. Pharmaceutical Dissolution Testing Services Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 239.10 million
Revenue forecast in 2033
USD 423.71 million
Growth rate
CAGR of 7.41% from 2025 to 2033
Actual data
2018 - 2024
Forecast period
2025 - 2033
Quantitative units
Revenue in USD million and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Method, dosage form, dissolution apparatus
Country scope
U.S.
Key companies profiled
Intertek Group Plc.; Avivia BV; Almac Group; Agilent Technologies, Inc.; Catalent, Inc.; Thermo Fisher Scientific Inc., Cambrex Corp; Charles River Laboratories; Boston Analytical; Pace Analytical Life Sciences; SOTAX; AMRI; SGS SA
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
U.S. Pharmaceutical Dissolution Testing Services Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the U.S. pharmaceutical dissolution testing services market report based on method, dosage form, and dissolution apparatus:
-
Method Outlook (Revenue, USD Million, 2021 - 2033)
-
In Vitro
-
In Vivo
-
-
Dosage Form Outlook (Revenue, USD Million, 2021 - 2033)
-
Capsule
-
Tablets
-
Others
-
-
Dissolution Apparatus Outlook (Revenue, USD Million, 2021 - 2033)
-
Basket
-
Paddle
-
Others
-
Frequently Asked Questions About This Report
b. The U.S. pharmaceutical dissolution testing services market size was estimated at USD 224.36 million in 2024 and is expected to reach USD 239.10 million in 2025.
b. The U.S. pharmaceutical dissolution testing services is expected to grow at a compound annual growth rate of 7.41% from 2025 to 2033 to reach USD 423.71 million by 2033.
b. In-vitro segment dominated the U.S. pharmaceutical dissolution testing services market with a share of 58.09% in 2024. This is attributable to its cost-effectiveness, regulatory acceptance, and widespread use in early-stage drug development and quality control.
b. Some key players operating in the U.S. pharmaceutical dissolution testing services market include Intertek Group Plc.; Avivia BV; Almac Group; Agilent Technologies, Inc.; Catalent, Inc.; Thermo Fisher Scientific Inc., Cambrex Corp; Charles River Laboratories; Boston Analytical; Pace Analytical Life Sciences; SOTAX; AMRI; SGS SA
b. Key factors that are driving the market growth include rising demand for generic drugs and bioequivalence studies, constant regulatory emphasis on consistent drug release profiles and quality control, and growing outsourcing activities
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










