- Home
- »
- Market Trend Reports
- »
-
Cell & Gene Therapy Clinical Trials: Current Dynamics & Pipeline Outlook
Report Overview
The cell & gene therapy clinical trials landscape is witnessing rapid growth, driven by increasing genetic and rare diseases, accelerating regulatory pathways, rising venture capital investment,growing demand for outsourced cell & gene therapy services & rising advancements in gene editing and vector delivery technologies are enabling more precise, durable therapies, expanding the therapeutic pipeline and global trial footprint.
Cell and gene therapies are transforming modern medicine by providing innovative treatment options for a variety of conditions, such as cancer, rare genetic disorders, and blood-related diseases. The therapies specific alter the genetic makeup of cells, either by correcting faulty genes or introducing new ones to target specific diseases. For instance, the Cancer Atlas mentioned that there will be 29 million cancer cases by 2040. As the incidence of cancer continues to rise globally, there is an increasing demand for innovative and effective treatment options. Cell and gene therapies offer a promising avenue for treating various cancers by leveraging the patient's cells to target and eliminate cancerous cells. This demand for novel therapies is expected to drive the need for specialized expertise and infrastructure, boosting the cell and gene therapy CDMO market
In addition, cell & gene therapies (CGT) represent a major breakthrough in advanced treatment methods, with the potential to revolutionize care by addressing the underlying causes of various diseases at the cellular and genetic levels. These therapies are considered highly promising solutions for a wide array of conditions. Furthermore, therapies are increasingly becoming a substantial part of the drug development landscape worldwide. Moreover, growing demand for personalized medical solutions and significant advancements in gene-editing technologies are driving biotech companies to create therapies that provide notable benefits compared to traditional treatment approaches.
Cell & Gene Therapy Clinical Trials: Current Dynamics & Pipeline Outlook Report Coverage
Market Outlook
Prevalence Trends Analysis
R&D Investment & Funding Analysis
Industry Ecosystem Analysis
Market Dynamics
Regulatory Framework
List of Top 200 Active Trials by Phase, Sponsor, and Indication
Emerging Clinical Trial Model Analysis
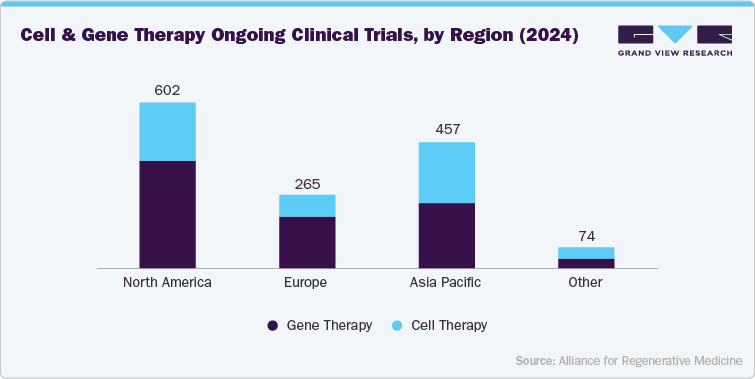
Global Cell & Gene Therapy Clinical Trials, by Phase & Study Design
Global Cell & Gene Therapy Clinical Trials, by key Indications, By Region
Some other emerging trends such as advancements in technology, changing regulatory frameworks, and an increasing global disease burden contribute to growing focus on innovating drugs and devices propelling cell & gene therapy clinical trials. The advancements in technology are significantly enhancing the progress of cell and gene therapy clinical trials by developing therapies safer, quicker, and precise. Besides, innovations in gene editing technologies such as CRISPR, base editing, and prime editing have transformed precision medicine, allowing for the correction of disease-related mutations directly at the DNA level. Moreover, in cell therapy, the shift from autologous to allogeneic approaches including CAR-T, CAR-NK, and TCR-T therapies has improved scalability and decreased manufacturing times. Furthermore, delivery technologies like AAV and lentiviral vectors, along with new non-viral systems such as lipid nanoparticles, have enhanced both transgene expression and safety. Digital innovations, including AI-driven trial design, decentralized monitoring, and integration of real-world data, optimizing patient recruitment and the tracking of long-term outcomes. In addition, modular manufacturing systems and closed-loop processing ensure consistent, GMP-compliant product development. Thus, these advancements reduce trial costs, shorten development timelines, and facilitate regulatory approval by generating robust, real-time data. As technology continues to progress, it will remain integral to the acceleration and success of CGT clinical trials.
Moreover, regulatory frameworks for cell and gene therapy (CGT) are adapting to meet the unique challenges presented by these innovative treatments. Organizations such as the FDA, EMA, and PMDA have established expedited pathways, including RMAT in the U.S., PRIME in the EU, and Sakigake in Japan to accelerate the development of therapies for chronic health conditions. These regulations focus on early collaboration with developers, rolling submissions, and adaptive trial designs. Furthermore, regulatory bodies mandate long-term follow-up to evaluate safety and efficacy, particularly for therapies that are intended to be administered just once. Standardized guidelines concerning vector safety, chemistry, manufacturing, and controls (CMC), as well as post-marketing surveillance, are essential for securing approval and ensuring patient access to CGT. Such factors are expected to drive the market over the estimated time period.
Venture Funding and Partnership Analysis
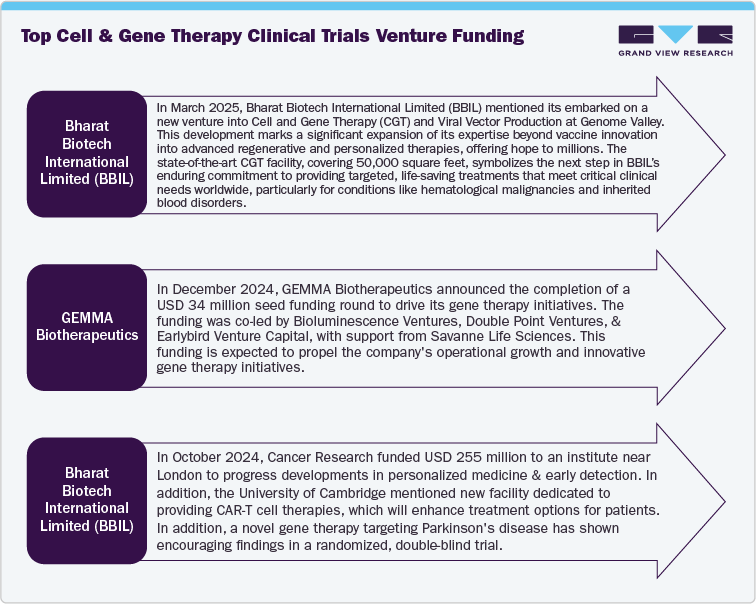
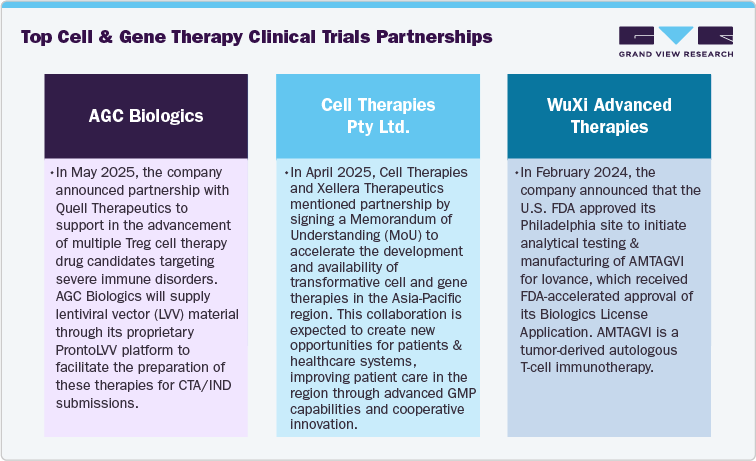
Venture funding and partnerships in CGT clinical trials market are driven by growing number investors particularly interested in unique technologies such as allogeneic CAR-T, in vivo gene editing, and advanced delivery systems that demonstrate strong preclinical results and regulatory approval. Besides, strategic collaborations among biotech and pharmaceutical companies are expected to increase the aimed at leveraging proprietary delivery methods, sharing the financial risk through co-funded clinical trials, and exploring applications beyond oncology, especially in neurology and hematology. Moreover, favorable regulatory incentives, such as RMAT and PRIME, along with real-world value assessments have supported to boost the investment. Furthermore, the Asia-Pacific region is emerging as a prominent hub for CGT investment, particularly in the areas of autoimmune and genetic disorders. Thus, expanding funding and partnerships are expected to support the market growth over the estimated time period.
Cell & Gene Therapy Pipeline Analysis
The cell and gene therapy pipeline is evolving rapidly due to technological advances in gene editing such as CRISPR, and base editing improved viral and non-viral delivery platforms like AAV, and LNPs, and the growing shift toward scalable allogeneic cell therapies. Increased regulatory support, such as RMAT and Fast Track designations, accelerates clinical timelines. Rising investor and strategic partnerships among biotech and pharma further drive innovation and trial expansion. In addition, expanding therapeutic focus beyond oncology into rare, neuromuscular, and autoimmune diseases is diversifying the pipeline, making CGT more commercially viable and clinically impactful across global healthcare markets.
Cell & Gene Therapy Pipeline Analysis for Targeted therapeutic areas
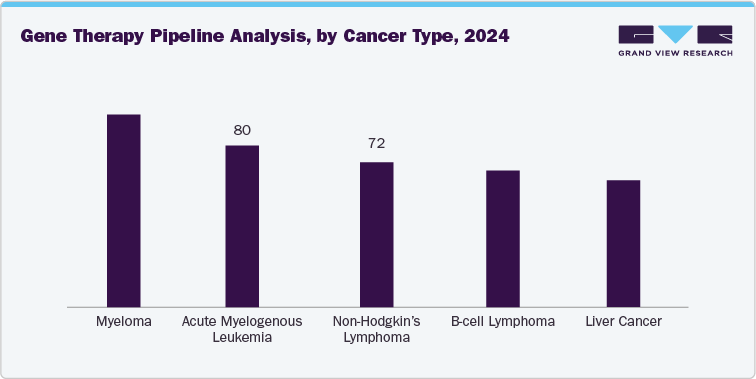
Cell Therapy Pipeline Analysis: According to the Gene, Cell, & RNA Therapy Landscape Report mentioned in April 2025, there are currently 966 non-genetically modified cell therapies in development, representing 22% of the overall gene, cell, & RNA therapies. Besides, oncology & rare diseases are anticipated to remain as major focus areas for these therapies. In addition, among the non-genetically modified cell therapies in the preclinical to pre-registration phases for rare diseases, 62% are focused on non-oncological rare conditions. In addition, the report mentioned key therapeutic targets within cell therapy, including myeloma, acute myelogenous leukemia, non-Hodgkin's lymphoma, B-cell lymphoma, and liver cancer, which contribute to an expanding pipeline.

Gene Therapy Pipeline Analysis: According to the Gene, Cell, & RNA Therapy Landscape Report mentioned in April 2025, there were 2,154 gene therapies including genetically modified cell therapies like CAR-T in development as of Q1 2025. In addition, there are 1,070 gene therapies at various stages from preclinical to preregistration that are specifically targeted at rare diseases. Besides, eight of the top ten rare diseases in this category are oncological. Furthermore, liver cancer has emerged as a new addition to the five rare diseases for which gene therapies are being developed, along with myeloma, acute myelogenous leukemia, non-Hodgkin’s lymphoma, and B-cell lymphoma, among others.
Gene Therapy in pre-registration:
Gene Therapies that are currently in the pre-registration phase are nearing the final step before obtaining regulatory approval. These treatments in pre-clinical registration ideally have successfully completed all necessary clinical trial phases and have demonstrated positive safety and efficacy results. Thus, the companies submit detailed data packages to regulatory bodies such as the FDA or EMA for evaluation. This phase involves assessments of clinical outcomes, manufacturing quality, and the long-term effects on patients. Therapies in pre-registration attract significant attention from investors, healthcare providers, and patients, as they are poised to enter the market soon. During this stage, the emphasis is on ensuring regulatory compliance, finalizing labeling, discussing pricing, and preparing for the commercial launch. Some examples of pre-registration gene therapy are as follows:
Country
Company
Gene Therapy
U.S.
Rocket Pharmaceuticals
RP-L201
Abeona
Pz-cel
Neurotech Pharmaceuticals
NT-501
Ultragenyx
UX111
Replimune
vusolimogene oderparepvec
Precigen
PRGN-2012
Europe
Rocket Pharmaceuticals
RP-L102
China
Belief BioMed
BBM-H901
Helixmith
donaperminogene seltoplasmid
Chongqing Precision Biotech
pulkilumab (pCAR-19B) cells
South Korea
Curocell
Anbal-cel
Ecosystem Analysis of the Cell & Gene Therapy Clinical Trials Market
The ecosystem of cell & gene therapy clinical trials is rapidly changing driven by an array of stakeholders and specialized infrastructure. At the forefront biotech and pharmaceutical companies lead the innovation and the development of clinical trials. These sponsors work closely with academic institutions, research hospitals, and contract research organizations (CROs) to design, execute, and manage trial data. In addition, contract development and manufacturing organizations (CDMOs) play a critical role for the production of cell and gene products, ensuring compliance with strict regulatory standards.
Moreover, regulatory agencies such as the FDA, EMA, and NMPA offer accelerated pathway for the approval of trials, particularly aiming for rare and life-threatening conditions. Besides, funding from investors and venture capital supports the early stages of development, while partnerships between large pharmaceutical companies and emerging biotech companies promote late-stage trials and the pathways to commercialization. Technology providers contribute tools for gene editing, vector development, and digital trial management. Patient advocacy groups and real-world data providers play a pivotal role in shaping trial design, participant recruitment, and measuring outcomes, particularly for rare diseases. This ecosystem is increasingly global, with North America, Europe, and Asia-Pacific serving as key centers for trials. Effective collaboration among all participants is crucial for promoting the safe, scalable, and equitable distribution of CGT therapies.
Emerging Clinical Trial Models
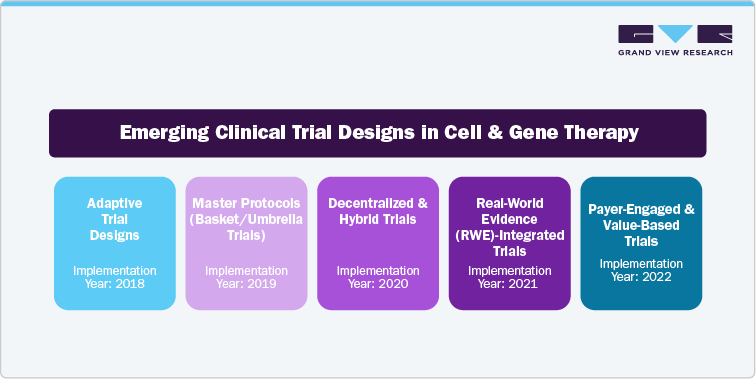
Innovative clinical trial models are reshaping the development, testing, and commercialization of cell and gene therapies. Traditional trial approaches often fail to adequately address the unique challenges posed by CGTs, such as limited patient populations, the need for long-term efficacy data, and manufacturing issues. As a result, the industry is increasingly embracing novel strategies, including decentralized and hybrid trial designs, to enhance patient access and retention, particularly in rare and pediatric conditions. Adaptive trial methodologies allow for real-time modifications to protocols based on interim findings, which speeds up decision-making processes. Master protocols enable the conduct of multi-arm studies across various indications or therapies, thereby streamlining research efforts. Furthermore, the incorporation of RWE facilitates long-term follow-up and meets regulatory requirements. Models that engage payers and focus on value-based outcomes are also being developed to align trial results with health economics and reimbursement standards. These advancements, introduced between 2018 and 2022, are essential for mitigating risks in clinical development, enhancing regulatory approval chances, and ensuring the commercial success of CGTs in a rapidly evolving landscape.
Approved Cell & Gene Therapy (2024)
Approved cell and gene therapies represent a significant advancement in modern medicine, providing potentially curative treatments for previously untreatable or rare diseases. These therapies function by modifying or substituting defective genes or cells to restore their normal operation. While these approved therapies demonstrate long-term efficacy, there are still obstacles to overcome cost, scalability in manufacturing, and post-marketing safety monitoring.
Therapy
Originator company
Product
Generic Name
Disease
Location Approved
Gene Therapy
Autolus
Aucatzyl
obecabtagene autoleucel
Acute lymphocytic leukemia
U.S.
Pfizer
Beqvez
fidanacogene elaparvovec
Hemophilia B
Canada, U.S., EU
CARsgen Therapeutics
zevorcabtagene autoleucel
zevorcabtagene autoleucel
Relapsed or refractory multiple myeloma
China
Source: Grand View Research,Gene, Cell, & RNA Therapy Landscape Report
This report presents a comprehensive examination of active clinical trials across various phases, giving stakeholders a worldwide perspective on current research efforts. It highlights trends, major contributors, and the most promising investment opportunities. Through insights on the number of trials, sponsors, and indications at each stage, this table is an essential tool for tracking the advancement of the cell & gene therapy pipeline, enabling informed decisions regarding future investments and trial strategies.
List of Major Active Trials by Phase, Sponsor, and Indication, A Key Example:
Clinical Trial Study Title
CD19 CAR T-Cell Therapy for R/R Non-Hodgkin Lymphoma and Acute Lymphoblastic Leukemia
Study Status
Recruiting
Phase
Phase I
Study Type
Interventional
Sponsor
Vinmec Research Institute of Stem Cell and Gene Technology
Number of Patients (Enrollment)
16
Start Date
2/8/2023
Primary Completion Date
31/7/2025
Completion Date
31/7/2025
Locations
Vinmec Research Institute of Stem Cell and Gene Technology, Hanoi, 100000, Vietnam
Other aspects that shall be analyzed will include the market overview, clinical trials by study design, by key indications, by region, and list of key clinical trials, sponsors, among several other factors.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


