- Home
- »
- Clinical Diagnostics
- »
-
Bloodstream Infection Testing Market Size Report, 2030GVR Report cover
![Bloodstream Infection Testing Market Size, Share & Trends Report]()
Bloodstream Infection Testing Market (2023 - 2030) Size, Share & Trends Analysis Report By Product (Instruments, Reagents & Consumables), By Sample Type (Blood Culture), By Technology (PCR, ISH), By End-user, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-140-3
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Bloodstream Infection Testing Market Trends
The global bloodstream infection testing market size was valued at USD 689.13 million in 2022 and is estimated to grow at a compound annual growth rate (CAGR) of 4.5% from 2023 to 2030. Bloodstream infection testing is crucial in identifying the bacteria and other pathogens responsible for blood infections. In densely populated areas with minimal hygiene practices, the risk of spreading infectious diseases significantly threatens human health. This challenges public health and requires effective diagnostic solutions to detect and mitigate these infections. The increasing number of infectious diseases globally and the growing awareness and emphasis on infection control are some of the major factors driving the market.
The COVID-19 pandemic had a moderate impact on the market for bloodstream infection testing. A retrospective observational study conducted at a tertiary care center in Jaipur, India, aimed to assess the prevalence and spectrum of bloodstream infections in COVID-19 patients. During the 5-month study period, out of 1,578 COVID-19-positive patients admitted to the center, a total of 158 blood cultures were obtained from these inpatients through the Department of Microbiology. Among these cultures, 15 (9.4%) showed positive results.
Additionally, using invasive medical procedures and devices in COVID-19 patients has increased the risk of healthcare-associated bloodstream infections. On the positive side, the pandemic has accelerated the adoption of infection prevention measures and heightened the importance of effective diagnostic tools. As a result, the bloodstream infection market is witnessing increased demand for innovative solutions to address the challenges posed by COVID-19. However, the use of invasive medical procedures and devices in COVID-19 patients has increased the risk of healthcare-associated bloodstream infections. On the positive side, the pandemic has accelerated the adoption of infection prevention measures and heightened the importance of effective diagnostic tools. As a result, the bloodstream infection market is witnessing increased demand for innovative solutions to address the challenges posed by COVID-19.
Increasing government initiatives and increasing awareness about the importance of blood donation is expected to propel market growth. For instance, as per the data published by WHO, 119 countries highlight a significant growth of 10.7 million blood donations volunteered by unpaid donors between 2008 and 2018. The South-East Asia Region witnessed the highest increase in voluntary unpaid blood donations (127%), followed by the Region of the Americas (81%) and Africa (81%). As a crucial measure, the World Health Organization (WHO) strongly recommended mandatory screening of all blood donations for infections before their utilization. This screening includes HIV, hepatitis B, hepatitis C, and syphilis and should adhere to quality system requirements. Consequently, there is a growing demand for advanced screening technologies and solutions to effectively identify potential infections in donated blood, thereby driving the market.
Moreover, recent years have witnessed increasing cases of healthcare-associated BSI (HA-BSI), majorly caused due to the use of intravascular catheters. For instance, as per the World Health Organization, in 2019, resistant pathogens causing bloodstream infections emerged as the second leading cause of global burdens related to antimicrobial resistance (AMR), resulting in nearly 1.5 million fatalities worldwide. These infections have profound health implications for affected individuals. In Europe, healthcare-associated bloodstream infections (HA-BSI) are the second most prevalent cause of disability and premature deaths caused by healthcare-associated infections (HAIs). A broad study across 25 countries revealed a substantial 23.6% excess mortality rate in adult patients due to catheter-related bloodstream infections. These findings highlight the significant impact of HA-BSIs on patient outcomes and emphasize the urgent need for effective interventions in the healthcare sector. Similarly, the World Health Organization (WHO) report shows around 250,000 BSIs cases annually in the U.S., with approximately 80,000 ICU patients suffering from catheter-related BSIs. Hospitals and clinical diagnostics laboratories have a high demand for advanced products to detect these infections accurately.
Product Insights
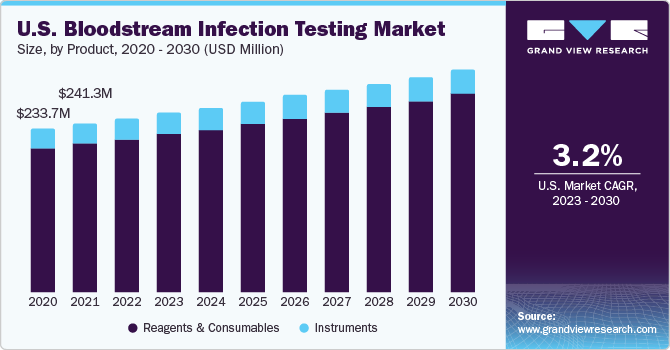
The reagents and consumables segment accounted for the largest revenue share of 88.12% in 2022. The segment is also expected to grow at the fastest rate over the forecast period. The segment's growth is driven by the easy availability and frequent purchase of screening products for donors and recipients. Moreover, the market is propelled by the wide range of blood grouping, typing, and donor screening reagents, kits, and assays offered by global and local manufacturers.
Additionally, the expanding product portfolio caters to the diverse needs of healthcare facilities, further fueling the growth of the segment in the bloodstream infection testing industry. For instance, in August 2022, a molecular diagnostic company, Immunexpress, Pty Ltd., launched SeptiCyte RAPID EDTA blood-compatible cartridges in European. The advanced SeptiCyte RAPID CE-IVD cartridge is a first-to-market hosts response technology which adds undiluted EDTA blood as a validated sample type demonstrating a significant development.
Sample Type Insights
The blood culture segment accounted for the largest revenue share of 74.80% in 2022 and is expected to witness the fastest CAGR over the forecast period. Blood culture tests are essential for diagnosing various infections, including bacterial, fungal, and mycobacterial infections. Both manual and automated methods are used by healthcare professionals to detect these infections.
Moreover, despite being a proven method, blood culture testing has continued to evolve with technological advancements. The introduction of automated blood culture systems, improved bottle designs, and faster detection methods have enhanced the efficiency and turnaround time of results, further solidifying the dominance of the blood culture segment. For instance, in March 2022, Accelerate Diagnostics, Inc., an in-vitro diagnostics company, launched the Accelerate Arc system. It comprised the Arc Module and BC Kit, an automated path to accurate and rapid microbial recognition for positive blood cultures.
Technology Insights
The PCR segment held the largest revenue share of 59.7% in 2022 and is expected to grow with the fastest CAGR over the forecast period. The most used nucleic acid amplification method for identifying infections directly from blood and positive blood cultures is the polymerase chain reaction (PCR). Either multiplex PCR or broad-range PCR is the basis for the PCR tests utilized in diagnostic labs. Increasing demand for advanced bloodstream infection testing methods, rising number forensic & research laboratories, and growing prevalence of diseases, such as chronic diseases, infectious diseases, are among the major factors expected to drive the PCR market growth.
Moreover, the introduction of technologically advanced PCR tests, government initiatives, increasing prevalence of targeted diseases, and application expansion of existing technologies are expected to drive the segment over the forecast period. For instance, in June 2021, Accelerate Diagnostics, a biotech company based in Tucson, Arizona, USA, was granted a funding award of up to USD 578,000 by CARB-X. The funds are specifically allocated for developing innovative fiber optic technology aimed at diagnosing sepsis or assessing the risk of sepsis. This financial support signifies a significant opportunity for the company to advance its sepsis diagnostic capabilities and contribute to the improvement of patient outcomes.
End-user Insights
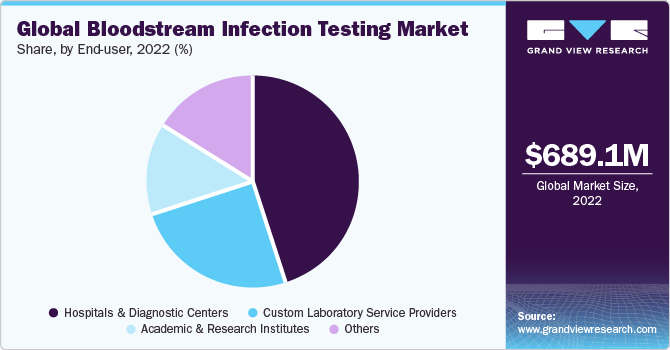
The hospital and diagnostic centers segment accounted for the largest revenue share of 44.84% in 2022 and is expected to witness the fastest CAGR from 2023 to 2030. Hospitals are at the forefront of diagnosing and treating bloodstream infections, which continue to pose a significant healthcare challenge. With the rise in hospital-acquired infections and sepsis cases, there is an increasing need for accurate and timely bloodstream infection testing to guide appropriate treatment decisions. This drives the demand for bloodstream infection testing services in hospitals. In addition, healthcare facilities, including hospitals, strongly emphasize infection control and patient safety. Implementing strong bloodstream infection testing protocols helps identify infections early, prevent their spread, and enhance patient outcomes.
Hospitals often collaborate with diagnostics laboratories and testing providers to streamline and enhance their bloodstream infection testing capabilities. Such collaborations leverage the expertise and resources of both entities, leading to improved diagnostic accuracy, expanded testing capacity, and better patient care. These partnerships fuel the growth of the market by driving demand for blood testing services.
Regional Insights
North America dominated the market and accounted for the largest revenue share of 44.68% in 2022. This is due to the region's Increasing disposable income, highly developed health infrastructure, favorable reimbursement policies, and increasing strategic initiatives and product launches by the major companies operating in the market. In October 2022, BD, a global medical technology company, and Magnolia Medical Technologies, Inc. entered a commercial agreement. The collaboration aims to assist U.S. hospitals in reducing blood culture contamination, enhancing testing accuracy, and ultimately improving clinical outcomes. Therefore, such initiatives with effective tools and technologies enhance the reliability and efficiency of blood culture testing, thereby boosting the demand for such testing methods in the region.
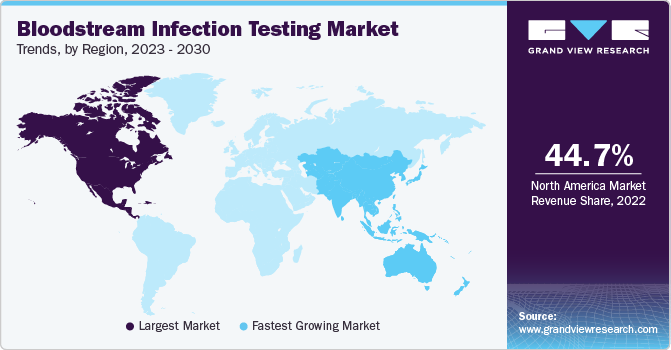
Asia Pacific is expected to witness the fastest CAGR over the forecast period. Technological advancements and rising government initiatives are expected to increase the demand for the bloodstream infection testing market in the region. For instance, in May 2023, the state government of Orissa, India, decided to expand the NAT-PCR testing facility to all blood centers in a comprehensive manner rather than in phases. This expansion was estimated to cost approximately Rs 200 crore (USD 24.3 Million). This decision came in response to two PILs urging the introduction of NAT-PCR facility in all blood banks in India due to its ability to detect HIV-1, HIV-2, hepatitis B, and hepatitis C at an earlier stage than the traditional ELISA test. Currently, 11 blood banks in the state have adopted the NAT-PCR technology, accounting for 47% of the total blood collections. The remaining 45 blood collection centers are expected to be equipped with NAT-PCR testing facilities by the end of 2025 to enhance the testing capabilities further. This expansion will significantly improve the efficiency and accuracy of blood testing, ensuring early detection of infectious diseases.
Key Companies & Market Share Insights
The key companies operating in bloodstream infection testing are attempting to enhance their product portfolio by upgrading their products and exploring acquisitions and government authorizations to increase their client base and obtain a larger market share. Furthermore, the key players in bloodstream infection testing are implementing strategies such as partnerships, mergers and acquisitions, product and service launches, joint ventures, agreements, expansion, and collaboration, to strengthen their position in the market. For instance, in July 2022, T2 Biosystems, Inc., a key company operating in early detecting sepsis-causing antibiotic resistance genes and pathogens, received the U.S. Food and Drug Administration (FDA) grant for its T2Lyme Panel. This panel will allow professionals to ensure patients receive suitable therapy faster and help in preventing the negative impact of treatment and the overuse of antibiotics. Some of the major players in global bloodstream infection testing market include:
-
bioMérieux SA
-
BD
-
Cepheid.
-
Seegene Inc.
-
T2 Biosystems, Inc.
-
F. Hoffmann-La Roche Ltd
-
Siemens Healthcare Limited
-
Luminex Corporation.
-
Bruker
-
Accelerate Diagnostics, Inc.
Bloodstream Infection Testing Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 717.93 million
Revenue forecast in 2030
USD 977.57 million
Growth rate
CAGR of 4.5% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD million and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, sample type, technology, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
bioMérieux SA; BD; Cepheid.; Seegene Inc.; T2 Biosystems, Inc.; F. Hoffmann-La Roche Ltd; Siemens Healthcare Limited; Luminex Corporation.; Bruker; Accelerate Diagnostics, Inc.
Customization scope
Free report customization (equivalent to up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional, and segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Bloodstream Infection Testing Market Report Segmentation
This report forecasts global, regional, and country revenue growth and analyzes the latest industry trends in each sub-segment from 2018 to 2030. For this study, Grand View Research has segmented the global bloodstream infection testing market report based on product, sample type, technology, end-user, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Reagents & Consumables
-
Instruments
-
-
Sample Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Whole Blood
-
Blood Culture
-
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
PCR
-
Mass Spectroscopy
-
In Situ Hybridization
-
Others
-
-
End-User Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals & Diagnostic Centers
-
Custom Laboratory Service Providers
-
Academic & Research Institutes
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East and Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global blood stream infection testing market size was estimated at USD 689.13 million in 2022 and is expected to reach USD 717.93 million in 2023.
b. The global blood stream infection testing market is expected to grow at a compound annual growth rate of 4.51% from 2023 to 2030 to reach USD 977.57 million by 2030.
b. The reagents and consumables segment dominated the global market in 2022 and captured the maximum share of the overall revenue. The segment's growth is driven by the easy availability and frequent purchase of screening products for donors and recipients. Moreover, the market is propelled by the wide range of blood grouping, typing, and donor screening reagents, kits, and assays offered by global and local manufacturers.
b. Some key players operating in the blood stream infection testing market include bioMérieux SA, BD, Cepheid., Seegene Inc., T2 Biosystems, Inc., F. Hoffmann-La Roche Ltd, Siemens Healthcare Limited, Luminex Corporation., Bruker, Accelerate Diagnostics, Inc.
b. The increasing number of infectious diseases globally and the growing awareness and emphasis on infection control are some of the major factors driving the global bloodstream infection testing market.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










