- Home
- »
- Biotechnology
- »
-
mRNA Synthesis Raw Materials Market Size Report, 2033GVR Report cover
![mRNA Synthesis Raw Materials Market Size, Share & Trends Report]()
mRNA Synthesis Raw Materials Market (2026 - 2033) Size, Share & Trends Analysis Report By Type (Capping Agents, Nucleotides, Plasmid DNA, Enzymes), By Application (Vaccine Production, Therapeutics Production), By End Use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-022-4
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2021 - 2024
- Forecast Period: 2026 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
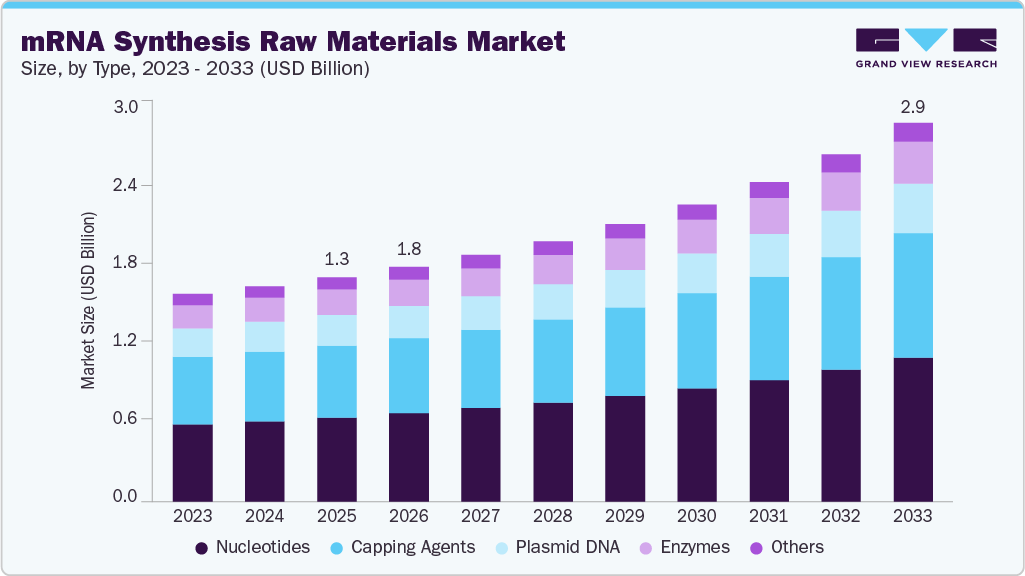
mRNA Synthesis Raw Materials Market Summary
The global mRNA synthesis raw materials market size was estimated at USD 1.73 billion in 2025 and is projected to reach USD 2.93 billion by 2033, growing at a CAGR of 7.10% from 2026 to 2033. The growing mRNA vaccine and therapeutic pipeline, higher investments in cell and gene therapy R&D, The increasing need for high-quality synthesis reagents, and the expansion of GMP-compliant manufacturing by biotechnology and pharmaceutical companies are the main drivers of this growth.
Key Market Trends & Insights
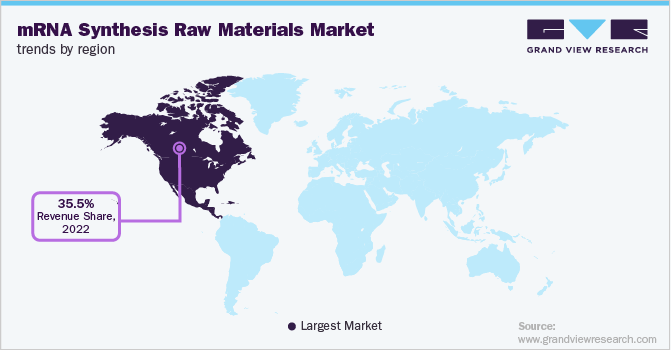
- North America mRNA synthesis raw materials market held the largest share of 36.0% of the global market in 2025.
- The mRNA synthesis raw materials industry in the U.S. is expected to grow significantly over the forecast period.
- By type, the nucleotides segment held the highest market share of 37.56% in 2025.
- Based on application, the vaccine production segment held the highest market share in 2025.
- By end user, the biopharmaceutical & pharmaceutical companies segment held the highest market share in 2025.
Market Size & Forecast
- 2025 Market Size: USD 1.73 Billion
- 2033 Projected Market Size: USD 2.93 Billion
- CAGR (2026-2033): 7.10%
- North America: Largest market in 2025
- Asia Pacific: Fastest growing market
Growth of mRNA Vaccines and Therapeutics
The rapid development and global deployment of regulatory agency-approved mRNA vaccines during the COVID-19 pandemic demonstrated the scalability and effectiveness of mRNA technology. Authorizations and approvals from agencies such as the FDA, EMA, and WHO validated mRNA as a safe and commercially viable platform, accelerating its acceptance for large-scale immunization programs and future therapeutic applications. Large-scale production by companies such as Moderna and Pfizer-BioNTech significantly increased demand for critical raw materials, including enzymes, modified nucleosides that enhance mRNA stability and reduce immunogenicity, and high-purity nucleotides to support sustained and expanding global vaccination efforts.
Regulatory agency-approved mRNA vaccines
Brand (Generic) name
Approval year
Regulatory agency
Comirnaty (Tozinameran)
2020
EMA
2020
HC
2020
MHRA
2021
FDA
Comirnaty Original/Omicron BA.4-5 (Tozinameran/Famtozinameran)
2022
EMA
2022
HC
2022
MHRA
Spikevax (Elasomeran)
2020
HC
2021
EMA
2021
MHRA
2022
FDA
Spikevax Bivalent Original/Omicron BA.1 (Elasomeran/Imelasomeran)
2022
EMA
2022
MHRA
Spikevax Bivalent Original/Omicron BA.4-5 (Elasomeran/Davesomeran)
2022
EMA
Source: Journal of Biomedical Science, Secondary Research, Grand View Research
The expanding use of mRNA therapeutics in cancer, rare genetic disorders, and other diseases is accelerating market growth, supported by a robust development pipeline that requires reliable GMP-grade raw materials. For instance, in April 2024, a partnership between CureVac and MD Anderson Cancer Center to develop off-the-shelf mRNA cancer vaccines highlights the rising demand. Moreover, ongoing innovations in mRNA synthesis technologies are increasing the need for advanced and specialized reagents, making mRNA vaccine and therapeutic development a key market driver.
Technological Improvements in mRNA Synthesis Technology
The demand for specialized raw materials, such as chemically modified nucleotides such as pseudo uridine and 5-methylcytidine that improve stability, translation efficiency, and immune tolerance, is being driven by advancements in mRNA synthesis technologies. For instance, in March 2025, Telesis Bio obtained up to USD 21 million to develop its Gibson SOLA technology for quick DNA and mRNA synthesis, enabling the production of high-quality nucleic acids on-site. High-performance enzymes and reagents are also becoming more necessary to achieve higher yield and purity due to advancements in enzymatic synthesis and in vitro transcription protocols.
Technological progress extends beyond synthesis to include improved delivery systems, such as lipid nanoparticles (LNPs), which protect mRNA and facilitate cellular uptake. Specialty lipids and excipients that must adhere to strict quality and regulatory standards are also necessary for the production of these delivery vehicles. The demand for complex raw materials has increased throughout the whole supply chain for mRNA production because of these technological advancements.
Market Concentration & Characteristics
The degree of innovation in the mRNA synthesis raw materials industry is significant, with new technologies and processes constantly being developed to improve efficiency and effectiveness. The mRNA field is expected to evolve rapidly, with ongoing research leading to discoveries and applications. For instance, in April 2024, Moderna and OpenAI partnered to integrate generative AI into Moderna’s operations to accelerate mRNA therapeutic development.
Mergers and acquisition activities can significantly impact the growth of the mRNA synthesis raw materials industry. Partnerships help companies to reduce costs and increase efficiency. In February 2022, Merck KGaA acquired Exelead for around USD 780 million in cash to reinforce its life sciences business segment. The initiative expanded the company’s life sciences portfolio and improved its market presence.
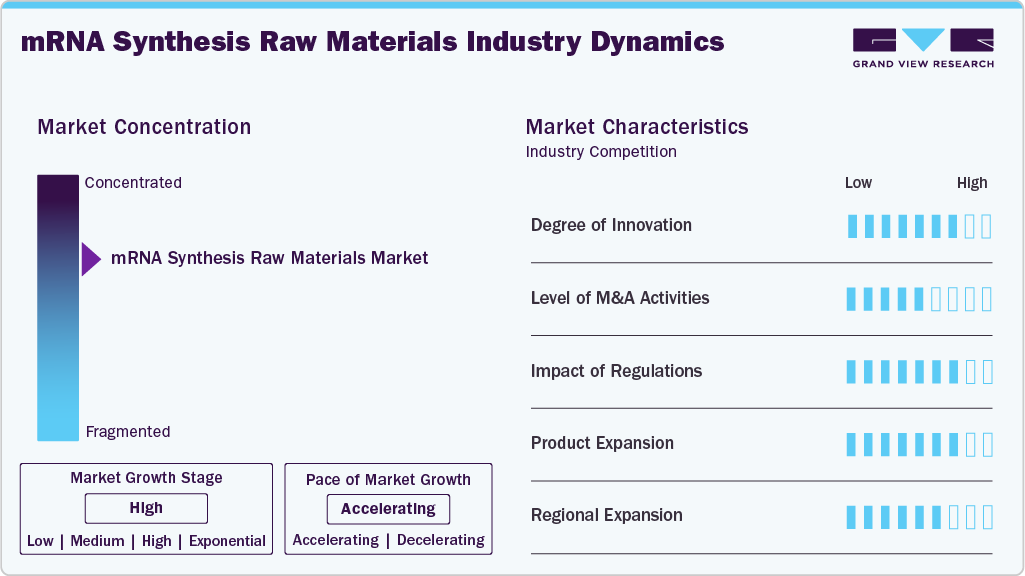
The safety, efficacy, and quality aspects are the focus of the regulatory framework for mRNA products, and they have been very stringent. The approvals of the mRNA cancer vaccine produced by BioNTech and utilizing a new modified nucleotide, which was approved by the FDA, illustrate the necessity of raw materials that comply with the regulations. Adherence to the good manufacturing practices (GMP) and acceptance of the FDA or European Medicines Agency (EMA) requirements not only facilitates but also shortens the time for approvals, and thus, the patients can access new mRNA therapies faster.
The mRNA synthesis raw materials industry is experiencing growth due to the expanding applications of mRNA technology, advancements in biotechnology & production processes, and increased research and development investments. mRNA technology allows for the development of personalized treatments tailored to individual genetic profiles. For instance, in May 2025, a personalized mRNA-based CRISPR therapy was successfully administered to a baby with a rare genetic disorder, highlighting the growing demand for mRNA synthesis raw materials as personalized medicine advances.
The mRNA synthesis raw materials industry has witnessed significant regional expansion in recent years. Companies are expanding their manufacturing capacities to meet the growing global demand for mRNA products. This includes establishing new production facilities and scaling up existing ones in various regions. For instance, in September 2022, TriLink BioTechnologies expanded its GMP-grade mRNA raw material portfolio with the launch of N1-Methyl-Pseudouridine-5’-Triphosphate, a key modified nucleotide for mRNA manufacturing.
Type Insights
The nucleotides segment dominated the global industry in 2025, accounting for 37.56% of total revenue. The segment’s growth is driven by rising demand for high-purity nucleotides for mRNA vaccines, therapeutics, and research, reflecting their critical role in ensuring mRNA stability and sequence fidelity. Advances in nucleotide modification technologies and expanding biopharmaceutical pipelines are further accelerating adoption.
The capping agents segment is expected to register the fastest CAGR over the forecast period as market players are advancing capping technologies to support mRNA therapeutics and vaccine development. For example, in May 2025, TriLink BioTechnologies launched an mRNA synthesis kit featuring Capping technology to enable higher yields and lower dsRNA content.
Application Insights
The vaccine production segment held the largest revenue market share at 83.96% in 2025. This can be attributed to the rapid production of mRNA vaccines compared to the conventional vaccines that utilize cell-culture-based production paths, which are often prone to failure. For instance, in April 2024, IDT Australia and Sanofi entered into a Master Service Agreement for the cGMP manufacture of Sanofi's RNA vaccines at IDT's Melbourne facility.
The therapeutics production segment is expected to expand at a significant CAGR during the forecast period due to the increasing prevalence of various chronic and infectious diseases. For instance, according to data published by the World Health Organization (WHO), 1.25 million people died from tuberculosis (TB) in 2023. mRNA-based therapeutics present promising treatment strategies for several diseases but also help in prevention. Hence, there is an increase in clinical trials and in vivo models to produce various therapeutics based on mRNA platforms, which will likely aid revenue generation in this segment. For instance, in September 2024, Primrose Bio and ExPLoRNA Therapeutics formed a strategic partnership to co-develop RNA polymerases and capping compounds, which aim to improve mRNA quality, manufacturing efficiency, and therapeutic efficacy in response to rising demand for mRNA synthesis raw materials.
End use Insights
The biopharmaceutical and pharmaceutical companies segment dominated the market, accounting for 49.23% of revenue in 2025. The growth of the market is driven by strong commercial success and rising demand for therapeutics. An expanding drug development pipeline has increased the need for mRNA-based raw materials. For instance, in June 2025, BioNTech and Bristol Myers Squibb partnered on BNT327, a Phase 3 bispecific cancer antibody, highlighting rising demand for mRNA synthesis raw materials.
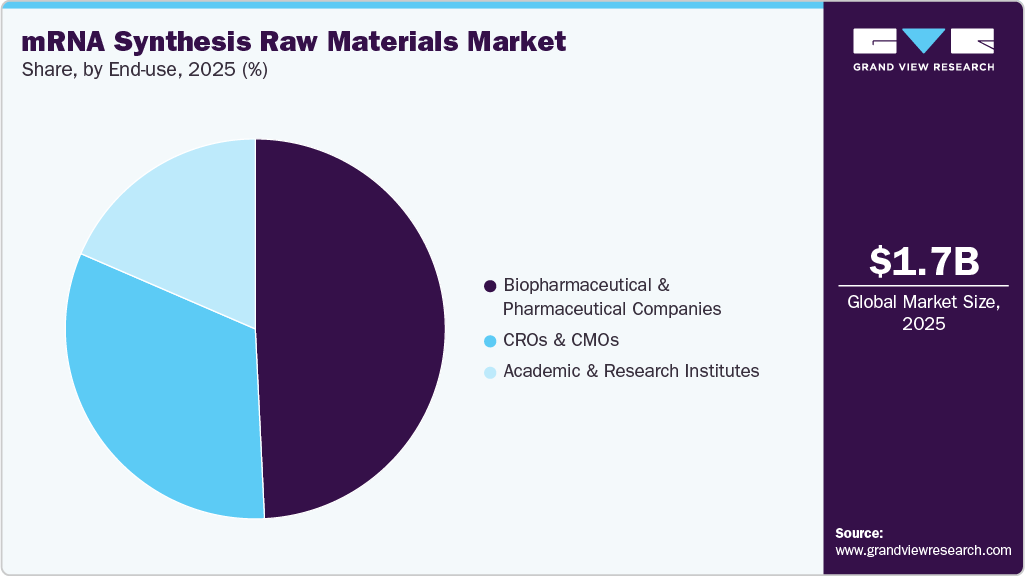
The CROs & CMOs segment is expected to grow rapidly, driven by biopharmaceutical companies outsourcing mRNA R&D and clinical trials to reduce costs and accelerate timelines. CROs’ specialized expertise, advanced technologies, and scalable mRNA synthesis services are fueling this demand.
Regional Insights
North America dominated the global mRNA synthesis raw materials industry in 2025 and accounted for the largest share of 36.00% of the overall revenue. The presence of local players, such as Providence Therapeutics, which offers personalized mRNA vaccines to treat chronic diseases, including cancer, is expected to aid revenue generation in the region. Several innovations by local players in the area are also expected to drive the mRNA synthesis raw materials market to a certain extent.
U.SmRNA Synthesis Raw Materials Market Trends
The U.S. mRNA synthesis raw materials market is projected to experience growth, backed by solid research funding, major industry participants, and strong healthcare infrastructure. For instance, in August 2023, Applied DNA Sciences launched its Linea IVT Evaluation Kit, streamlining in vitro transcription and advancing mRNA synthesis technology, further driving market growth.
Europe mRNA Synthesis Raw Materials Market Trends
Europe's mRNA synthesis raw materials market was identified as a lucrative region in this industry. The strong presence of well-established and emerging product manufacturers and the continuous organic and inorganic growth strategies they adopt are expected to create a lucrative environment for market growth. Offerings can be adapted for research use only (RUO) or manufactured at GMP grade, supporting the advancement of mRNA-based therapeutic innovations.
The mRNA synthesis raw materials market in the UK held a significant share in 2025. Growing government deals with companies active in the mRNA space are being signed within the country to manage the pandemic. For instance, in July 2024, UK-based CDMO Touchlight signed a non-exclusive license agreement with GSK to use its proprietary enzymatic doggybone DNA (dbDNA) technology for mRNA vaccine manufacturing.
The mRNA synthesis raw materials market in Germany is anticipated to grow significantly over the forecast period. For instance, in February 2023, BioNTech announced new facility expansion in Marburg, Germany, to produce plasmid DNA, a vital building block for mRNA-based drugs, which further expands the demand in the country.
Asia Pacific mRNA Synthesis Raw Materials Market Trends
The Asia Pacific mRNA synthesis raw materials market held a fastest CAGR of 7.55% over the forecast period. With rising healthcare spending and investments in the region, many raw material manufacturers are shifting operations to the Asia Pacific countries, which is contributing to the growing demand for the market. For instance, in November 2024, Singapore launched the NATi mRNA BioFoundry, Asia's first automated mRNA facility, advancing nucleic acid therapeutics through automation, machine learning, and the Wellcome Leap R3 Program.
The mRNA synthesis raw materials market in China is expected to grow, driven by rising demand for mRNA drugs and vaccines. For instance, in February 2022, Walvax Biotechnology and Honeywell launched China’s first-ever digital mRNA vaccine manufacturing unit in Yunnan, which will facilitate the speedy and large-scale production of vaccines for both local and international markets.
The Japan mRNA synthesis raw materials market is witnessing significant growth over the forecast period. Major players in the market are adopting several strategies, including mergers and acquisitions, partnerships, and collaborations, to stay competitive. For instance, in July 2024, Moderna and Mitsubishi Tanabe Pharma Corporation will jointly promote the mRNA respiratory vaccine portfolio of Moderna in Japan, thus pushing the mRNA production raw materials sector in the area even more.
MEA mRNA Synthesis Raw Materials Market Trends
The MEA mRNA synthesis raw materials market is expected to grow rapidly, supported by improving infrastructure, supportive regulatory policies, rising disposable incomes, and a growing geriatric population. For example, in February 2025, TriLink BioTechnologies and Avantor entered a distribution partnership that aimed at increasing the availability of nucleic acid products in the EMEA region, improving the supply chain, and speeding up the development of mRNA therapy.
Kuwait's mRNA synthesis raw materials market is anticipated to experience growth over the forecast period. Several multinational and regional pharmaceutical companies are expected to be active in the Kuwaiti market, particularly offering mRNA vaccines during winter when the risk of seasonal flu increases. This seasonal demand will likely drive higher consumption of raw materials required for mRNA synthesis, thereby boosting the country's overall market growth.
Key mRNA Synthesis Raw Materials Company Insights
The mRNA synthesis raw materials market is shaped by prominent players known for their robust product portfolios, advanced research capabilities, and strong global distribution networks. By providing high-purity reagents, enzymes, and nucleotides necessary for the production of good-quality mRNA, the leading firms F. Hoffmann-La Roche Ltd., Merck KGaA, Thermo Fisher Scientific, and Maravai LifeSciences have all secured their places in the market as main players. These firms continue to innovate through substantial R&D investments and strategic collaborations that support the rapidly growing field of mRNA-based therapeutics and vaccines.
New England Biolabs, Jena Bioscience GmbH, and Yeasen Biotechnology enhance their market position through the invention of specific enzymes, modified nucleotides, and other key elements needed for in vitro transcription processes. Their ability to supply GMP-grade materials and scalable clinical and commercial manufacturing solutions has made them key partners in mRNA drug development pipelines.
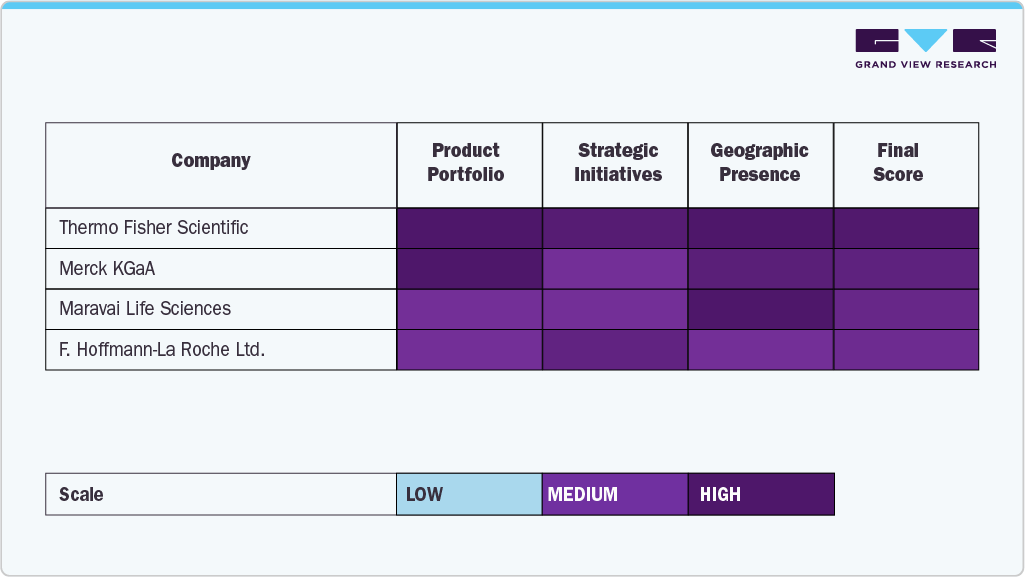
Strategically investing, innovating their technology, and customizing their products for mRNA vaccine makers and contract manufacturers, Creative Biogene, BOC Sciences, and HONGENE, the new entrants, are especially present in the market. These players are essential in overcoming the major hurdle of large-scale mRNA production by providing a reliable and cost-efficient supply chain for the raw materials.
Key mRNA Synthesis Raw Materials Companies:
The following are the leading companies in the mRNA synthesis raw materials market. These companies collectively hold the largest market share and dictate industry trends.
- F. Hoffmann-La Roche Ltd.
- Jena Bioscience GmbH
- Merck KGaA
- Yeasen Biotechnology (Shanghai) Co., Ltd.
- BOC Sciences
- Thermo Fisher Scientific, Inc.
- Maravai LifeSciences
- New England Biolabs
- Creative Biogene
- HONGENE.
Recent Developments
-
In November 2025, in the United States, Moderna invested USD 140 million to complete its domestic mRNA manufacturing network, strengthening local supply chains and boosting demand for mRNA synthesis raw materials.
-
In November 2024, Syvento Biotech and Cytiva inaugurated a FlexFactory facility in Poland to accelerate mRNA therapy development. The modular platform integrates automation and digital tools, enhancing production efficiency and scalability. This collaboration bolsters regional manufacturing capabilities and expedites patient access to innovative treatments.
-
In September 2024, Hongene Biotech Corporation and ReciBioPharm announced a strategic partnership to enhance gene editing drug manufacturing. This collaboration integrates Hongene's proprietary sgRNA synthesis technology into ReciBioPharm's facilities in Watertown, Massachusetts, enabling comprehensive services, including plasmid, mRNA, sgRNA, LNP, and fill-finish capabilities under one roof. The alliance aims to streamline the development and manufacturing process, reducing logistical complexities and accelerating drug development timelines.
-
In February 2022, Merck KGaA, Darmstadt, Germany, completed its acquisition of Exelead, a U.S.-based CDMO. The deal aimed to enhance Merck's mRNA vaccine and therapeutic development capabilities. Merck committed to investing in scaling up Exelead's technology over the next ten years.
mRNA Synthesis Raw Materials Market Report Scope
Report Attribute
Details
Market size value in 2026
USD 1.81 billion
Revenue forecast in 2033
USD 2.93 billion
Growth rate
CAGR of 7.10% from 2026 to 2033
Base year for estimation
2025
Historical data
2021 - 2024
Forecast period
2026 - 2033
Quantitative units
Revenue in USD million and CAGR from 2026 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, application, end user, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, India, China, Japan, Australia, South Korea, Thailand, Brazil, Argentina, Saudi Arabia, UAE, South Africa, Kuwait
Key companies profiled
F. Hoffmann-La Roche Ltd.; Jena Bioscience GmbH; Merck KGaA; Yeasen Biotechnology (Shanghai) Co., Ltd.; BOC Sciences; Thermo Fisher Scientific, Inc.; Maravai LifeSciences; New England Biolabs; Creative Biogene; HONGENE
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
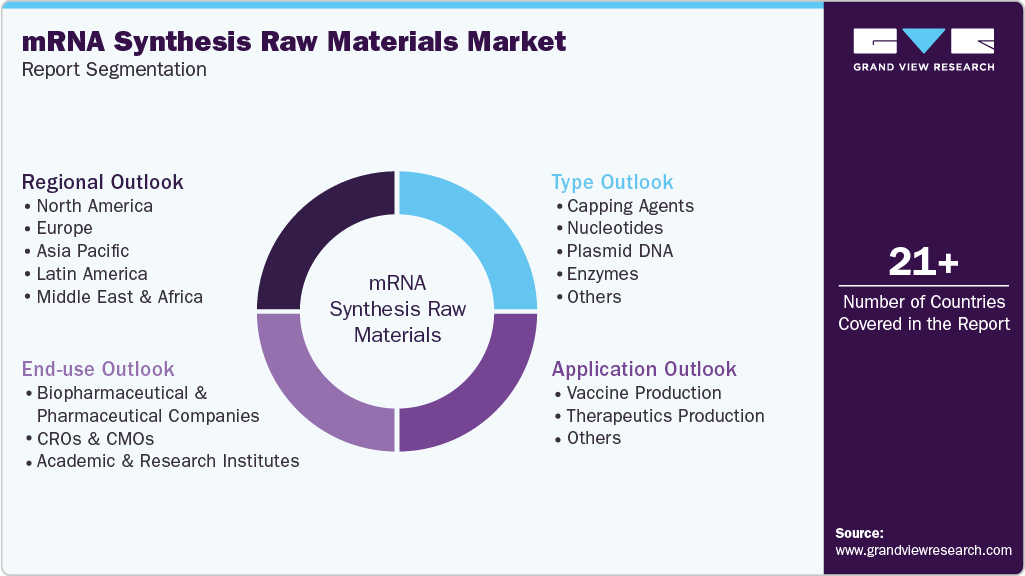
Global mRNA Synthesis Raw Materials Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2021 to 2033. For this report, Grand View Research has segmented the mRNA synthesis raw materials market on the basis of type, application, end user, and region:
-
Type Outlook (Revenue, USD Million, 2021 - 2033)
-
Capping Agents
-
Nucleotides
-
Modified Nucleic Acids
-
N1-methylpseudouridine-triphosphate
-
5-Methylcytidine triphosphate (5mCTP)
-
-
Natural Nucleic Acids
-
Adenine
-
Guanine
-
Cytosine
-
Uracil
-
-
-
Plasmid DNA
-
Enzymes
-
Polymerase
-
RNase Inhibitor
-
DNase
-
Others
-
-
Others
-
-
Application Outlook (Revenue, USD Million, 2021 - 2033)
-
Vaccine Production
-
Therapeutics Production
-
Others
-
-
End use Outlook (Revenue, USD Million, 2021 - 2033)
-
Biopharmaceutical & Pharmaceutical Companies
-
CROs & CMOs
-
Academic & Research Institutes
-
-
Regional Outlook (Revenue, USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global mRNA synthesis raw materials market is expected to grow at a compound annual growth rate of 7.10% from 2026 to 2033 to reach USD 2.93 billion by 2033.
b. The capping agents segment held the significant share of 32.0% in 2025 owing to the advancements in the capping technology and their applications in the biopharma industry and research.
b. Some key players operating in the mRNA synthesis raw materials market include F. Hoffmann-La Roche Ltd.; Jena Bioscience GmbH; Merck KGaA; Yeasen Biotechnology (Shanghai) Co.,Ltd.; BOC Sciences; Thermo Fisher Scientific, Inc.: Maravai LifeSciences; New England Biolabs; Creative Biogene; and HONGENE
b. The major factors driving the market growth include, growing academic and industrial interest in mRNA technology, advantages of mRNA vaccines and, increasing funding for research. In addition, the increasing therapeutic applications of RNA technology are projected to offer lucrative opportunities for the mRNA synthesis raw materials market during the forecast period.
b. The global mRNA synthesis raw materials market size was estimated at USD 1.73 billion in 2025 and is expected to reach USD 1.81 billion in 2026.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










