- Home
- »
- Medical Devices
- »
-
Neuroendoscopy Devices Market Size & Share Report, 2030GVR Report cover
![Neuroendoscopy Devices Market Size, Share & Trends Report]()
Neuroendoscopy Devices Market (2025 - 2030) Size, Share & Trends Analysis Report By Product (Rigid, Flexible), By Application (Transnasal, Intraventricular, Transcranial), By Usability, By End Use, By Region, And Segment Forecasts
- Report ID: GVR-2-68038-081-1
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Neuroendoscopy Devices Market Trends
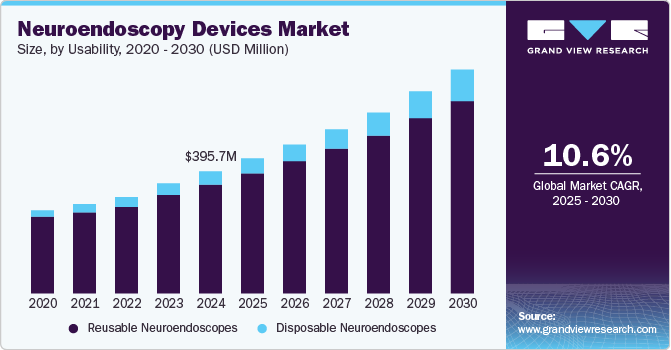
The global neuroendoscopy devices market size was valued at USD 395.73 million in 2024 and is projected to grow at a CAGR of 10.63% from 2025 to 2030, owing to the rising incidence of brain tumors and advancement in healthcare technologies. Moreover, the rising demand for minimally invasive surgical devices by healthcare professionals drives the market. In addition, neuroendoscopy is the most preferred surgery for brain tumors and other nervous system-related diseases. For instance, according to the National Brain Tumor Society, in the U.S., an estimated number of 79,340 adults aged 40 and above are expected to be diagnosed with a primary brain tumor in 2023.
The neuroendoscopy devices market has witnessed a notable surge in FDA approvals and the launch of innovative products, driven by advancements in medical technology and an increasing demand for minimally invasive procedures. Innovations in imaging technology enhance the precision and effectiveness of diagnostic and therapeutic interventions, leading to a wider range of options for healthcare providers and improved patient outcomes. For instance, in August 2024, ClearMind Biomedical Inc. received U.S. FDA 510(k) clearance for its Neuroblade neuroendoscopy system. This system facilitates minimally invasive procedures with integrated illumination, visualization, suction, irrigation, powered debridement, and coagulation.
Recent developments in technologies and advancements in neuroendoscopy devices are assisting surgeons and doctors in conducting brain and central nervous system-related surgeries. Innovative and advanced neuroendoscopy devices offer convenience, driving market growth. Furthermore, emerging technology and software systems assisting healthcare professionals, such as artificial intelligence and robots, are anticipated to drive market growth.
Neurological conditions are increasingly recognized as a major public health challenge. A recent study highlighted by WHO indicates that over one in three people globally are affected by neurological conditions, making these disorders the leading cause of illness and disability worldwide. This rising prevalence of neurological disorders, including epilepsy, hydrocephalus, stroke, degenerative spine disease, dementia, & migraines, poses significant challenges for healthcare systems and is driving innovation and demand in the neuroendoscopy devices market. For instance, according to The Leagrave Therapy Clinic UK, the percentage rate of severe degenerative disc disease rises to around 60% in 70-year-olds.
Furthermore, favorable government initiatives and reimbursement policies fuel the market growth. For instance, the Japanese health finance system depended on Fee For Service (FFS), wherein reimbursement was offered under a single country-wide schedule, which enabled the Japanese health finance system to contain brief information about prescribed drugs, procedures, and diagnostics.
Market Concentration & Characteristics
The neuroendoscopy devices market is characterized by a high degree of innovation due to technological advancements in the endoscopes field, which led to a rise in the adoption of neuroendoscopy devices in the healthcare sector. Moreover, the advancements in endoscopic technologies, including high-definition visualization and the integration of artificial intelligence (AI) for enhanced diagnosis, boost the market growth.
The neuroendoscopy devices market is characterized by a medium level of merger and acquisition (M&A) activity. These M&A enable access to complementary technologies, expertise, and distribution channels, facilitating companies to capture a larger market share. For instance, in June 2024, KARL STORZ Endoscopy-America, Inc. announced its merger agreement with Asensus Surgical.
Neuroendoscopy devices require FDA clearance to ensure their safety and efficacy standards. Basic regulatory requirements for the manufacturing and distribution of medical devices in the U.S. are establishing registration, listing of medical devices, Investigational Device Exemption (IDE) for clinical research, Premarket Approval (PMA) process, labeling requirements, regulating Quality System (QS), and Medical Device Reporting.
Several market players are expanding their business by entering new business geographies to strengthen their market position and expand their product portfolio. For instance, in January 2021, ClearMind Biomedical Inc. received the U.S. FDA clearance for its Axonpen novel neuroendoscope system.
Product Insights
By product, the flexible neuroendoscopes segment dominated the neuroendoscopy devices market in 2024 with a revenue share of 64.1% and is expected to grow at the fastest rate over the forecast period. The growth of this segment is fueled by the rising prevalence of neurological conditions and the superior benefits offered by flexible neuro endoscopes compared to traditional surgical methods. For instance, according to the National Brain Tumor Society, around 700,000 individuals in the U.S. are living with a primary brain tumor, highlighting the need for effective diagnostic tools and innovative treatment approaches.
The rigid neuroendoscopes segment is expected to grow at a significant rate over the forecast period. A rigid neuroendoscope is a specialized instrument designed for examining and navigating the brain, characterized by its robust metal tube construction that offers advanced lenses and a light channel. This innovative device plays a crucial role in various neurosurgical procedures, such as tissue sampling from brain tumors, fluid removal from the brain's ventricles, and the treatment of conditions such as obstructive hydrocephalus, craniosynostosis, and skull base tumors. One of the major benefits of rigid neuroendoscopes is that they offer a high-quality clarity image that enhances visualization of inaccessible parts of the brain. Thus, such advantages provided by the neuroendoscopes boost the market growth.
Application Insights
By application, the transcranial segment dominated the neuroendoscopy devices market in 2024 with a revenue share of 38.6% and is anticipated to grow at the fastest rate over the forecast period. As the demand for precise and less invasive surgical techniques grows, transcranial neuroendoscopy is gaining traction. The ability to perform complex procedures with enhanced accuracy and reduced complication rates propels the adoption of these devices in surgical settings. With ongoing advancements in neuroendoscopic technology, the transcranial segment is expected to witness significant growth, driven by a shift toward minimally invasive approaches in neurosurgery.
The intraventricular segment in the neuroendoscopy devices market is anticipated to witness significant growth over the forecast period. The growing prevalence of hydrocephalus, particularly in pediatric populations, is driving increased demand for intraventricular neuroendoscopy devices. For instance, according to an article published by NCBI in August 2023, the global prevalence of hydrocephalus is around 85 per 100,000 people, with a significant difference between various age groups, such as 11 per 100,000 in adults and 88 per 100,000 in the pediatric population.
Usability Insights
By usability, the reusable segment dominated the neuroendoscopy devices market in 2024 with a revenue share of 89.2%, owing to low maintenance costs and rising demand for reusable neuroendoscopes. Reusable endoscopes are more robust and offer superior image quality due to their complex optical systems. In addition, reusable neuroendoscopes are more cost-effective and provide greater environmental sustainability by reducing medical waste.
The disposable segment in the neuroendoscopy devices market is anticipated to witness significant growth over the forecast period. Disposable neuroendoscopy devices are rapidly gaining popularity in the healthcare landscape, primarily due to their convenience and reduced risk of cross-contamination. These single-use instruments are designed for one-time application, eliminating the complexities and time associated with cleaning and sterilization.
End Use Insights
By end use, the outpatient facilities segment dominated the neuroendoscopy devices with a revenue share of 54.3% in 2024 and is anticipated to grow at the fastest rate over the forecast period, owing to the rising number of neuroendoscopic surgeries and the growing popularity of Ambulatory Surgical Centers (ASCs). ASCs are modern healthcare facilities designed for outpatient facilities and are well equipped to perform minimally invasive surgeries such as brain tumors and other nervous system-related surgeries. Further, faster patient recovery and shorter hospital stays are expected to accelerate the growth of outpatient facilities during the forecast period.
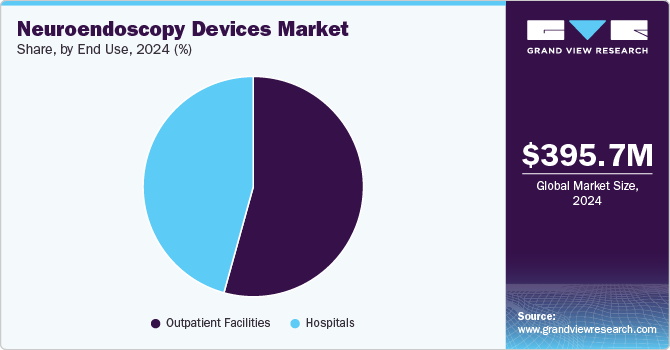
The hospitals segment in the neuroendoscopy devices market is expected to register significant growth over the forecast period. The rising disposable income, increasing health insurance coverage, and the rising number of neurosurgeons drive the growth. In addition, there are approximately 72,967 neurosurgeons globally. Neuroendoscopy is a complex surgery that requires highly trained neurosurgeons, and these skilled surgeons are usually found in well-equipped hospitals with the necessary resources.
Regional Insights
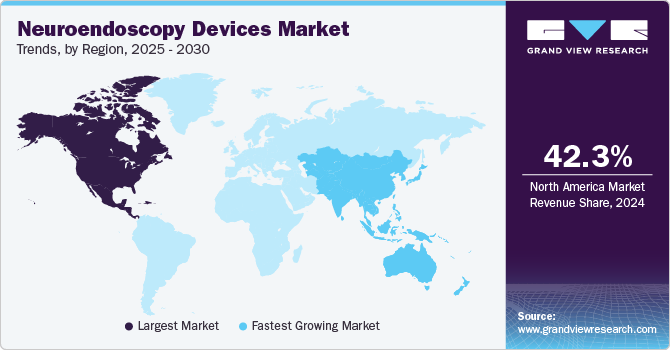
North America neuroendoscopy devices market dominated with a revenue share of 42.3% in 2024 and is expected to witness the fastest growth rate over the forecast period, owing to the presence of favorable government initiatives and highly developed healthcare infrastructure. The rising capital expenditure and health insurance coverage in countries such as the U.S. and Canada is driving the region growth. For instance, health insurance coverage in the U.S. and Canada were around 90% & 70%, respectively, in 2023.
U.S. Neuroendoscopy Devices Market Trends
The neuroendoscopy devices market in the U.S. held the largest market share in 2024. The presence of well-developed healthcare infrastructure and advanced neuroendoscopic devices drives the market. The rise in the number of brain tumors in the country is accelerating the growth. For instance, according to the National Brain Tumor Society, statistics of 2023, 1 million Americans were expected to be diagnosed with brain tumors, and approximately 18,990 were expected to die from a malignant brain tumor in 2023.
Europe Neuroendoscopy Devices Market Trends
Europe neuroendoscopy devices market is anticipated to register a considerable growth rate during the forecast period. Favorable macro-environment factors drive key players to revise their market entry strategies through mergers & acquisitions and technological collaborations to expand their footprint. For instance, in January 2021, Olympus Corporation signed an agreement to acquire Quest Photonic Devices B.V. for USD 60.18 million (EUR 50 million). Quest Photonic Devices B.V., a Netherlands-based company, offers Fluorescence Imaging Systems (FIS) for open and minimally invasive procedures.
Germany neuroendoscopy devices market is anticipated to register a considerable growth rate during the forecast period. Factors such as cost reduction, time efficiency, and the need to control endoscopy-related infections are driving the demand for disposable neuroendoscopy devices. Single-use endoscopes eliminate the costs associated with sterilization procedures, leading to overall cost reduction in endoscopy procedures, as highlighted by KARL STORZ, a manufacturer of disposable endoscopes in Germany.
Neuroendoscopy devices market in UK is anticipated to register a considerable growth rate during the forecast period the increasing number of patients suffering from intraventricular hemorrhage and brain tumors that require minimally invasive surgical procedures for diagnosis & treatment is contributing to market growth. According to the NHS report, over 12,000 individuals receive a diagnosis of a primary brain tumor in the UK annually, with approximately half being cancerous.
Asia Pacific Neuroendoscopy Devices Market Trends
The Asia Pacific region is anticipated to witness the fastest growth in the neuroendoscopy devices market over the forecast period. Key market players are developing strategies for expanding their business in this region. Growth in the region is expected to be driven by favorable initiatives undertaken by private players, such as training healthcare professionals and increasing R&D investments to develop advanced neuroendoscopes.
Japan neuroendoscopy devices market dominated in 2024. The high prevalence of neurological diseases and consistent product advancements are some factors expected to drive the demand for neuroendoscopy procedures. In Japan, GBM is an uncommon cancer, representing 1.68 cases per 100,000 individuals annually. In addition, increasing demand for early diagnosis and treatment of diseases is expected to boost the market.
Latin America Neuroendoscopy Devices Market Trends
Latin America is witnessing steady growth in the neuroendoscopy devices market, owing to the growing preference for minimally invasive surgeries over open surgeries, and increasing awareness about the use of neuroendoscopes for various diagnostic & therapeutic procedures is expected to drive the market. This region offers lucrative growth opportunities for the medical device industry.
Argentina neuroendoscopy devices market is anticipated to register a considerable growth during the forecast period. The availability of training centers for educating healthcare professionals regarding recent advancements in neuroendoscopes is boosting market growth. Moreover, continuous improvements in imaging and navigation systems further enhance the precision & safety of neurosurgical procedures, driving market growth.
Middle East & Africa Neuroendoscopy Devices Market Trends
The Middle East and Africa region are experiencing lucrative growth in the neuroendoscopy devices market. The market is gaining immense traction due to the increase in the number of hospitals, the growing geriatric population, and the rising demand for minimally invasive procedures.
South Africa neuroendoscopy devices market is anticipated to register a considerable growth rate during the forecast period. South Africa has a well-developed healthcare sector focused on providing advanced medical services and technologies to its population. The need to deliver high-quality healthcare services, accurate diagnoses, and minimally invasive treatments is increasing the demand for neuroendoscopes in South Africa.
Key Neuroendoscopy Devices Company Insights
Key participants in the neuroendoscopy devices market are focusing on developing innovative business growth strategies in the form of product portfolio expansions, partnerships & collaborations, mergers & acquisitions, and business footprint expansions.
Key Neuroendoscopy Devices Companies:
The following are the leading companies in the neuroendoscopy devices market. These companies collectively hold the largest market share and dictate industry trends.
- Karl Storz
- B. Braun SE
- Olympus Corporation
- Medtronic
- Ackermann Instrumente GmbH
- adeor medical AG
- Stryker
- Clarus Medical LLC
- Schindler Endoskopie Technologie GmbH
- Machida Endoscope Co., Ltd.
Recent Developments
-
In October 2023, Joimax GmbH introduced iLESSYS biportable, interlaminar endoscopic surgical system at EUROSPINE 2023 and the SMISS Annual Forum to treat spinal disorders.
Neuroendoscopy Devices Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 437.61 million
Revenue forecast in 2030
USD 725.62 million
Growth rate
CAGR of 10.63% from 2025 to 2030
Historical data
2018 - 2024
Forecast data
2025 - 2030
Report updated
December 2024
Quantitative units
Revenue in USD million/million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, application, usability, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; and MEA
Country scope
U.S.; Canada; Mexico; Germany; UK; Spain; Italy; France; Norway; Denmark; Sweden; Japan; China; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Karl Storz; B. Braun SE; Olympus Corporation; Medtronic; Ackermann Instrumente GmbH; adeor medical AG; Stryker; Clarus Medical LLC; Schindler Endoskopie Technologie GmbH; Machida Endoscope Co., Ltd.
Customization scope
Free report customization (equivalent up to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Neuroendoscopy Devices Market Report Segmentation
This report forecasts revenue growth at global, regional, and country level and provides an analysis on industry trends in each of the sub segments from 2018 to 2030. For this study, Grand View Research, Inc. has segmented the global neuroendoscopy devices market report based on product, application, usability, end use, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Rigid Neuroendoscopes
-
Flexible Neuroendoscopes
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Transnasal
-
Intraventricular
-
Transcranial
-
-
Usability Outlook (Revenue, USD Million, 2018 - 2030)
-
Reusable
-
Disposable
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Outpatient Facilities
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
NorthAmerica
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
Spain
-
Italy
-
France
-
Denmark
-
Norway
-
Sweden
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Argentina
-
-
MEA
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global neuroendoscopy devices market size was estimated at USD 395.73 million in 2024 and is expected to reach USD 437.61 million in 2025.
b. The global neuroendoscopy devices market is expected to grow at a compound annual growth rate of 10.63% from 2025 to 2030 to reach USD 725.62 million by 2030.
b. the transcranial segment dominated the neuroendoscopy devices market in 2024 with a revenue share of 38.6% and is anticipated to grow at the fastest rate over the forecast period. As the demand for precise and less invasive surgical techniques grows, transcranial neuroendoscopy is gaining traction.
b. Some key players operating in the neuroendoscopy devices market include Karl Storz, B. Braun SE, Olympus Corporation, Medtronic, Ackermann Instrumente GmbH, adeor medical AG, Stryker, Clarus Medical LLC, Schindler Endoskopie Technologie GmbH, Machida Endoscope Co., Ltd.
b. Key factors that are driving the market growth include rise in the brain injuries, and neural surgeries coupled with adoption of minimally invasive procedures.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










