- Home
- »
- Medical Devices
- »
-
Regulatory Affairs Outsourcing Market Size Report, 2030GVR Report cover
![Regulatory Affairs Outsourcing Market Size, Share & Trends Report]()
Regulatory Affairs Outsourcing Market Size, Share & Trends Analysis Report By Services, By Category (Pharmaceuticals, Medical Devices), By Company Size, By Indication, By Product Stage, By End-use, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-1-68038-556-4
- Number of Report Pages: 275
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Regulatory Affairs Outsourcing Market Trends
The global regulatory affairs outsourcing market size was accounted for USD 6.63 billion in 2023 and is anticipated to grow at a compound annual growth rate (CAGR) of 8.3% from 2024 to 2030. Outsourcing regulatory affairs has become an increasingly important practice in the healthcare industry. An increase in geographical expansion activities by companies that aim for speedy approvals in local markets is expected to contribute to the adoption of outsourcing models for regulatory services. The outsourcing market for regulatory affairs is expanding rapidly due to increased R&D activities, rising preference for personalized medicine and biologics augmenting the volume of clinical trial applications and product registrations.
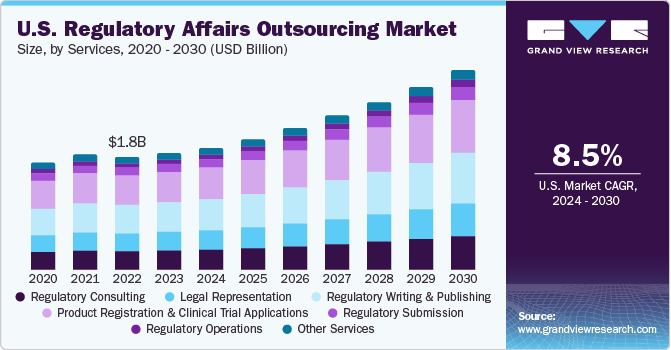
Further, healthcare companies are under constant pressure to procure timely clinical approvals from regulators in different nations. Such actions further promote the demand for regulatory affairs services, thus contributing to market growth. Also, increasing demand to obtain approval for new products while maintaining compliance, driving market progression. According to a survey sponsored by Genpact, 72.0% of executives from the life sciences industry consider regulatory compliance as one of the top three challenges life sciences companies face. Regulatory departments often face the burden of handling multiple tasks simultaneously and must ensure compliance with stringent regulatory standards. An increase in efforts by companies to expand their geographical reach and gain rapid approvals in global markets is expected to further contribute to the adoption of outsourcing models for regulatory services. High pressure on life sciences companies to reduce development costs further propels market growth opportunities. Growing usage of generics and demand for drugs & medical devices at affordable prices is expected to reduce healthcare costs, thereby boosting preference for regulatory affairs outsourcing.
Rising out-of-pocket expenditure among developed and developing countries, uneven economic growth, and measures taken by several governments to reduce the cost of drugs are a few factors contributing to the economic and competitive pressure, which, in turn, is expected to drive the demand for regulatory affairs outsourcing among life science companies. Product-specific clinical advice & strategy and regulatory compliance at the early stages of product development can be critical to product approval. Failure to address regulatory compliance in the early development stages often leads to delays in the approval process owing to inappropriately designed studies, manufacturing oversights, omitted studies, and other failures to meet the regulatory requirements.
Globalization of biopharmaceuticals and medical device companies is likely to be one of the major market drivers. Emerging markets from Asia Pacific, Latin America, and MEA regions offer low product development & manufacturing costs, tax benefits, and availability of skilled labor at relatively low costs with supportive regulations. The abovementioned factors have made the regional markets attractive prospects in terms of outsourcing and expansion for biopharmaceutical & medical device companies, thus stimulating the demand for regulatory services. Further, rising adoption of decentralized clinical trials, fast track approvals, and global harmonization in new product development are few other factors bolstering overall market demand.
Market Concentration & Characteristics
The market growth stage is medium, and the pace of market growth is accelerating. The regulatory affairs outsourcing market is characterized by a high degree of innovation, level of merger and acquisition strategies, impact of regulations, service expansion and regional expansion of market participants. Continuous advancements or upgradation in regulatory guidelines for better safety and efficacy, accelerating market demand.

Degree of Innovation
- The global regulatory affairs outsourcing market is characterized by a high degree of innovation. Growing integration of several advanced technologies such as Artificial Intelligence (AI), machine learning, Internet of Things (IoT), big data and analytics for regulatory affairs is likely to enhance overall market growth potential.
Level of M&A activities
- The level of M&A activities is expected to be at moderate level. Several market players, such as ICON Plc., Pharmalex GmbH, VCLS and IQVIA Inc., are involved in several merger and acquisition activities. M&A activities by major players aid in expanding their geographic reach and strengthening their market position.
Impact of Regulations
- The impact of Regulations is expected to be significant due to highly stringent regulatory compliance requirements. Rigorous regulatory standards are established to ensure that drugs and therapies undergoing testing are safe and effective. Changing regulatory guidelines for pharmaceutical and medical device products in different countries will boost market growth.
Service Expansion
- The key companies are implementing diverse strategic initiatives such as new service launches, expansions and partnerships to expand their service portfolio and gain a strong market foothold. Strong presence of skilled professionals to cater a wide range of services for precise outcomes and approvals is a key factor driving market demand.
Regional Expansion
- Growing initiatives by key market participants such as new facility expansions, partnership with local players to broaden their footprints in the emerging market. Rising need for regulatory compliance services and growing clinical trial services in the emerging regions such as Latin America and MEA offer expansion opportunities to the market players.
Service Insights
On the basis of service, the market is categorized into regulatory consulting, legal representation, regulatory writing & publishing, product registration & clinical trial applications, regulatory submissions, regulatory operations, and others. The product registration and clinical trial applications segment dominated the market and accounted for the largest revenue share of over 26.0% of the global revenue. The increasing number of clinical trials across the globe, stringent regulations in developed markets, and legal/regulatory reforms in emerging markets such as Asia Pacific are driving the outsourcing trend for clinical trial applications.
The legal representation services segment is anticipated to witness the fastest growth rate of 9.0% over the forecast period. The segment growth is owing to increasing demand for legal representatives worldwide on account of the globalization of medical devices and pharmaceutical companies. The regulations are very complex and ever-changing. The changing regulatory landscape in regions such as Asia Pacific, MEA, and Latin America increases the demand for local experts for legal representation for obtaining regulatory approvals and custom clearance. These factors are promoting the demand for legal representation services across the world.
Company Size Insights
On the basis of company sizes, the market has been divided into small, medium, and large. The medium-sized companies segment accounted for 46.7% of the revenue share in 2023 and is estimated to expand further, retaining the leading position over the forecast period. The presence of several mid-sized established providers, especially privately-held ones, is anticipated to contribute to this segment share. Moreover, medium-sized pharmaceutical and medical device companies do not have enough capital to develop an in-house regulatory affairs team, which is further driving the demand for regulatory affairs outsourcing among these companies.
The large companies’ segment is projected to register a significant growth rate of 7.7% over the forecast period. Large companies generally prefer to establish long-term relations with their service providers to avoid sudden disruption in their operations and thus, prefer a service provider that can meet their regulatory needs to support their various cross-scale and ramp-up operations. Apart from this, according to an article published by GEP (2020), large-scale pharma companies generally outsource about 50% of their regulatory affairs needs. These factors are contributing to the growth of this segment.
Category Insights
The category segment is subdivided into drugs, biologics, and medical devices. Drugs segment dominated the market and accounted for the largest share of 39.7% in 2023. The segment is currently leading the regulatory affairs outsourcing market due to the increasing demand for regulatory support services in the pharmaceutical industry. With the growing number of new drug applications and the increasing complexity of regulatory requirements, pharmaceutical companies are increasingly outsourcing their regulatory affairs functions to specialized service providers. Outsourcing enables these companies to access the expertise of regulatory professionals and reduce costs associated with maintaining an in-house regulatory affairs team. Additionally, the pharmaceutical segment is also witnessing growth due to the increasing focus on drug safety, efficacy, and quality, which requires extensive regulatory support.
However, the medical device segment is projected to register a considerable CAGR of 7.9% over the forecast period, which can be attributed to the fact that medical devices companies are now focusing on their core competencies and outsourcing noncore functions to increase their productivity and operational efficiency. The growing demand for advanced medical devices and new technological advancements in medical devices are further contributing to the growth of the segment.
Indication Insights
The oncology segment dominated the market and accounted for the largest share of more than 33.3% in 2023. The recent advances in the biology of cancer and the emergence of new tools for genome analysis have opened a clinical perspective in oncology, which has led to the development of personalized medicines. Scientific progress is driving an increase in the number of personalized medicine products and services, subject to regulatory review, hence contributing to market growth. Other indications included in the scope of the study are neurology, cardiology, immunology, and others.
The immunology segment is expected to expand at the fastest CAGR of 9.8% over the forecast period. This is owing to its potential in facilitating the treatment of various cardiovascular, neurological, oncological, and inflammatory diseases. The strategic initiatives undertaken by market players for immunology are anticipated to facilitate segment growth. For instance, in September 2021, Boehringer Ingelheim acquired Abexxa Biologics. This has broadened company’s cancer immunology and immunotherapeutics portfolio market. In addition, the COVID-19 pandemic created an urgent need for vaccines. Thus, the development of vaccines for COVID-19 is likely to have a positive impact on segment growth.
Product Stage Insights
The clinical studies segment dominated the market and accounted for the largest revenue share of 46.8% in 2023. This can be attributed to the increasing number of clinical trial registrations over the past few years. According to ClinicalTrials.gov, nearly 33,613 new trials were registered in 2023, as compared to around 32,540 in 2020. Moreover, this rise in the number of biologics, high demand for advanced technologies, and a requirement for personalized orphan drugs & medicines are other factors likely to fuel segment growth during the forecast period.
The preclinical segment is anticipated to expand at the fastest CAGR of 9.0% over the forecast period of 2024 - 2030. The rising demand for novel disease treatments, such as COVID-19, Zika virus, and Ebola, and the increasing prevalence of existing diseases, such as CVDs, cancer, and neurological diseases are the key factors contributing to the preclinical segment’s growth. Moreover, stringent regulations related to preclinical studies, laid down by global regulatory bodies, such as International Conference on Harmonization (ICH), WHO, FDA, EMEA (Europe), PMDA (Japan), ANVISA (Brazil), MHRA (UK), & ROEB (Canada), are further driving the demand for regulatory affairs outsourcing agencies for preclinical studies.
End-use Insights
The pharmaceutical companies segment dominated the market and accounted for the largest share of more than 40.3% in 2023. This segment growth is owing to the growth in evolving areas, such as biosimilar, orphan drugs, and personalized medicines, which are creating more demand for regulatory services, thereby boosting segment growth. In addition, the significant number of new drugs entering the pharmaceutical industry has further improved segment growth.
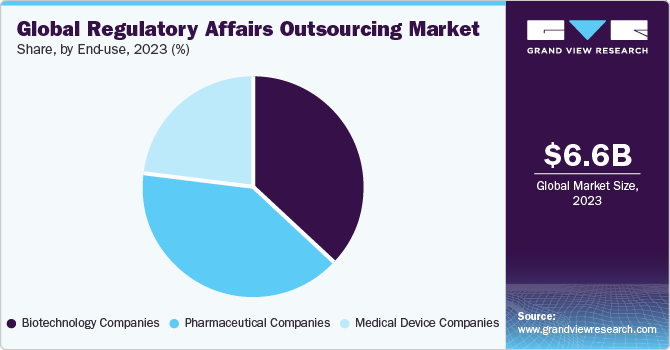
The medical device and biotechnology companies’ segments both have registered a substantial share in the market in 2023. This can be attributed to the increased demand for biopharmaceuticals, vaccines, advanced medical devices, and other products. The growing demand for wearable technologies, along with recent epidemic events, is further contributing to the substantial share of these segments. Furthermore, the increasing number of launches pertaining to medical devices is another significant factor boosting demand for regulatory affairs services, thus supporting segmental growth.
Regional Insights
The Asia Pacific region dominated the regulatory affairs outsourcing market and accounted for the largest revenue share of more than 39.9% in 2023. The region is also projected to witness the fastest CAGR over the forecast period. This can be attributed to the increasing number of clinical trials and the rising number of companies trying to enter markets in developing countries, such as India and China. Furthermore, the availability of a skilled workforce in the region at lower costs compared to the U.S. is another factor expected to propel the regional market growth.
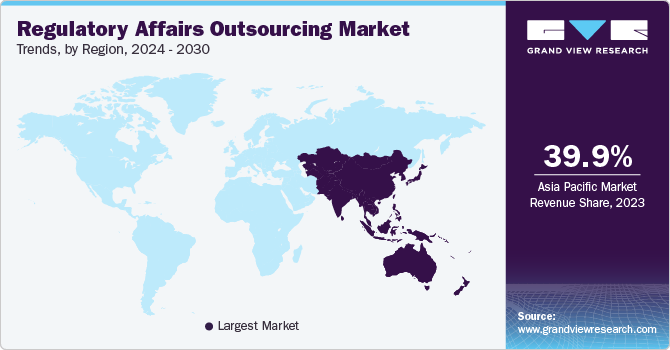
China is one of the most attractive markets for the biopharmaceutical industry. Growing geriatric population and presence of a large population belonging to the middle-income group are further increasing the demand for innovative and cost-effective medicines, which is expected to attract major biopharmaceutical and medical devices companies in this region. Seeking approval for new drugs is a primary challenge faced by biopharmaceutical companies as a clinical trial application process requires 12 to 24 months while approval process may require more than six years. Along with national approvals, the new drug requires approval at both provincial and city levels, which may require additional 4 to 5 years. Reforms in the healthcare sector in recent times are expected to streamline the approval process. Further, recent pricing reforms are expected to attract multinational companies, contributing to the demand for regulatory services. For instance, in January 2023, GenScript, which provides contract services to help other companies develop and manufacture biologic drugs, secured USD 224 million in funding from investors. This new investment can help GenScript's subsidiary, ProBio, expand its production capacity for biologic drugs, which are made using living cells and are often used to treat complex diseases including cancer & autoimmune disorders. ProBio's expansion plans include building new manufacturing facilities in China and overseas. Such initiatives are expected to propel the demand for regulatory affairs services in China.
The North America regional market also reported a significant share in the global industry. The presence of key pharmaceutical and medical device companies and the rise in R&D spending in the region are some of the key factors driving the market in North America. North America and Europe are expected to be the key markets for regulatory affairs outsourcing, owing to the presence of two major international regulatory agencies, the European Medicines Agency (EMA) and the U.S. FDA, which regulate more than half of the medical devices worldwide.
The U.S. accounts for a major share of clinical trial applications across the globe. Along with branded and patented products, there is a strong demand for cost-effective generic and biosimilar products in this region. For instance, in January 2022, around 33 FDA-approved biosimilars were available in the U.S., 21 of which were commercially available in the market. Thus, increasing development and approval of novel biosimilars is expected to propel the demand for regulatory services in the U.S., driving the market.
Key Regulatory Affairs Outsourcing Company Insights
The major players operating across the regulatory affairs outsourcing market are focused on the adoption of several strategic initiatives such as mergers, partnerships, acquisitions, etc. Companies such as Labcorp Drug Development, Charles River Laboratories, ICON plc, Genpact, WuXi AppTec, and Parexel International Corporation hold a notable share of the market. This is owing to their comprehensive service portfolio covering a wide range of regulatory affairs outsourcing services. Moreover, these companies are continuously investing in cutting-edge technologies for regulatory affairs to offer more accurate and efficient services. This technological advantage helps attract clients and contribute to a larger market share. The multinational presence of these companies coupled with implementation of strategic initiatives such as partnerships, mergers & acquisitions contribute to the company’s market presence.
Key Regulatory Affairs Outsourcing Companies:
The following are the leading companies in the regulatory affairs outsourcing market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these regulatory affairs outsourcing companies are analyzed to map the supply network.
- Accell Clinical Research, LLC
- Genpact
- CRITERIUM, INC
- Promedica International
- WuXi AppTec
- Medpace
- Charles River Laboratories
- ICON plc
- Labcorp Drug Development
- Parexel International Corporation
- Freyr
- PHARMALEX GMBH
- NDA Group AB
- Pharmexon
- Qvigilance
- BlueReg
- Cambridge Regulatory Services
- APCER Life Sciences, Inc.
- Real Regulatory Ltd
- VCLS
- PrimeVigilance
- ProPharma Group MIS Limited
- Regulatory Pharma Net srl
- ZEINCRO
- BioMapas
- REGENOLD GMBH
Recent Developments
-
In January 2023, AmerisourceBergen Corporation acquired PharmaLex Holding GmbH, a prominent service provider including regulatory affairs in the life sciences industry. This has broadened company’s service portfolio in a significant market.
-
In April 2022, VCLS collaborated with EC Innovations, a Chinese company that offers translation services globally and has vast experience translating highly regulated life sciences and medical content. The strategy helped to strengthen company’s offerings and operational capabilities.
Regulatory Affairs Outsourcing Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 7.03 billion
Revenue forecast in 2030
USD 11.31 billion
Growth rate
CAGR of 8.3% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Services, company size, category, indication, product stage, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Russia; Turkey; Netherlands; Switzerland; Sweden; Europe CIS Countries; Japan; China; India; Australia; South Korea; Indonesia; Malaysia; Singapore; Thailand; Taiwan; CIS Countries; Brazil; Mexico; Argentina; Colombia; Chile; South Africa; Saudi Arabia; UAE; Egypt; Israel; Kuwait
Key companies profiled
Accell Clinical Research; LLC; Genpact; CRITERIUM, INC; Promedica International; WuXi AppTec; Medpace; Charles River Laboratories; ICON plc; Covance, Inc. (Labcorp Drug Development); Parexel International Corporation; Freyr; PHARMALEX GMBH; NDA Group AB; Pharmexon; Qvigilance; BlueReg; Cambridge Regulatory Services; APCER Life Sciences, Inc.; Real Regulatory Ltd; VCLS; PrimeVigilance; ProPharma Group MIS Limited; Regulatory Pharma Net srl; ZEINCRO; BioMapas; Regenold GmbH
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Regulatory Affairs Outsourcing Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global regulatory affairs outsourcing market report based on services, company size, category, indication, stage, end-use, and region:
-
Service Outlook (Revenue, USD Million, 2018 - 2030)
-
Regulatory Consulting
-
Strategy & Development Planning
-
QA Consulting
-
Others
-
-
Legal Representation
-
Regulatory Writing & Publishing
-
Product Registration & Clinical Trial Applications
-
Regulatory Submission
-
Regulatory Operations
-
Other Services
-
-
Company Size Outlook (Revenue, USD Million, 2018 - 2030)
-
Small
-
Medium
-
Large
-
-
Category Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharmaceuticals
-
Regulatory Consulting
-
Strategy & Development Planning
-
QA consulting
-
Others
-
-
Legal Representation
-
Regulatory Writing & Publishing
-
Product Registration & Clinical Trial Applications
-
Regulatory Submissions
-
Regulatory Operations
-
Other Services
-
-
Medical Device
-
Regulatory Consulting
-
Strategy & Development Planning
-
QA Consulting
-
Others
-
-
Legal Representation
-
Regulatory Writing & Publishing
-
Product Registration & Clinical Trial Applications
-
Regulatory Submissions
-
Regulatory Operations
-
Other Services
-
-
-
Indication Outlook (Revenue, USD Million, 2018 - 2030)
-
Oncology
-
Neurology
-
Cardiology
-
Immunology
-
Others
-
-
Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
-
Preclinical
-
Clinical
-
PMA (Post Market Authorization)
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Medical Device Companies
-
Pharmaceutical Companies
-
Biotechnology Companies
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Russia
-
Turkey
-
Netherlands
-
Switzerland
-
Sweden
-
Europe CIS Countries
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Indonesia
-
Malaysia
-
Singapore
-
Thailand
-
Taiwan
-
CIS Countries
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
Colombia
-
Chile
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Egypt
-
Israel
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global regulatory affairs outsourcing market size was estimated at USD 7.0 billion in 2022 and is expected to reach USD 7.5 billion in 2023.
b. The global regulatory affairs outsourcing market is expected to witness a compound annual growth rate of 8.0% from 2023 to 2030 to, reach USD 12.82 billion by 2030.
b. Product registration & clinical trial applications held the largest share of 25.9% in 2022. The increasing number of clinical trials across the globe, stringent regulations in developed markets, and legal/regulatory reforms in emerging markets such as Asia Pacific is driving the outsourcing trend for clinical trial applications.
b. Some of the players operating in the regulatory affairs outsourcing market are Accell Clinical Research, LLC.; Genpact Ltd.; Criterium, Inc.; PRA Health Sciences; Promedica International; WuXi AppTec, Inc.; Medpace; Pharmaceutical Product Development, LLC (PPD); Charles River Laboratories International, Inc.; ICON plc; Covance, Parexel International Corporation; Inc.; and Freyr.
b. Key factors that are driving the regulatory affairs outsourcing market growth include an increase in geographical expansion activities by companies, an increase in the R&D activities, and rising clinical trial applications.
Table of Contents
Chapter 1 Methodology and Scope
1.1. Market Segmentation and Scope
1.2. Market Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. GVR’s Internal Database
1.5. Details of primary research
1.5.1. Data for primary interviews in North America
1.5.2. Data for primary interviews in Europe
1.5.3. Data for primary interviews in Asia Pacific
1.5.4. Data for primary interviews in Latin America
1.5.5. Data for Primary interviews in MEA
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.1.1. Approach 1: Commodity flow approach
1.7.2. Volume price analysis (Model 2)
1.7.2.1. Approach 2: Volume price analysis
1.8. Research Scope and Assumptions
1.8.1. List of Secondary Sources
1.8.2. List of Primary Sources
1.8.3. Objectives
Chapter 2 Executive Summary
2.1 Market Snapshot
2.2 Segment Snapshot
2.3 Competitive Landscape Snapshot
Chapter 3 Regulatory Affairs Outsourcing Market Variables and Trends
3.1 Market Lineage Outlook
3.2 Market Dynamics
3.2.1 Market Driver Impact Analysis
3.2.1.1 Changing Regulatory Landscape
3.2.1.2 Entry Of Companies In The Global Market
3.2.1.3 Life Science Companies Focusing On Their Core Competencies
3.2.1.4 Economic And Competitive Pressures
3.2.1.5 Demand For Faster Approval Process For Breakthrough Drugs And Devices
3.2.1.6 Growth In Emerging Areas Such As Personalized Medicine, Biosimilars, And Orphan Drugs
3.2.2 Market Restraint Impact Analysis
3.2.2.1 Risk Associated With Data Security
3.2.2.2 Monitoring Issues And Lack Of Standardization
3.3 Industry Challenges
3.3.1 MANAGING RELATIONSHIPS
3.4 Service Mapping, By Drug/Device Lifecycle
3.6 Service Price Analysis
3.5.1 Organization Structure
3.5.2 Price Level Analysis
3.5.2.1 Regulatory Affairs Service Price Analysis By Phase
3.5.2.2 Segment Category Level
3.5.3 Unit Level
3.5.4 Pricing Models
3.6 Technology Landscape
3.6.1 Existing And Emerging Use Of Technology And Preferred Tools
3.6.2 Differentiating In-House Tools Vs Widely Available
3.6.3 Tools That Have Seal Of Approval From Regulatory Authorities
3.6.4 Key Technology Pillars
3.6.4.1 Digital Transformation and Data Integrity
3.6.4.2 Artificial Intelligence, Machine Learning/Automation
3.6.4.2.1 Data Analysis and Predictive Modeling
3.6.4.2.2 Automation of Routine Tasks
3.6.4.2.3 Compliance Monitoring
3.6.4.2.4 Discovery and Development
3.6.4.2.5 Regulatory Guidelines Related to AI/ML:
3.6.4.2.5.1 FDA: Guidelines on AI/ML in Medical Devices
3.6.4.2.5.2 EMA: The Use of Artificial Intelligence (AI) in The Medicinal Product Lifecycle
3.6.4.2.5.3 MHRA: Guidance on Software and AI as a Medical Device
3.6.5 Big Data And Analytics
3.6.6 RTP, Text Processing And Analytics
3.6.7 Speech And Video Analytics
3.6.8 IoT (Internet Of Things)
3.6.8.1 Advanced Supply Chain Monitoring
3.6.9 AI Tools
3.6.9.1 ELICIT (Electronic Literature Control and Information Tracking)
3.6.9.2 HUMATA (Human and Machine Analysis of Texts and Arguments)
3.6.9.3 Chat GPT
3.6.9.4 Scite Assistant
3.6.9.5 Other AI Tools
3.7 Covid-19 Impact On Regulatory Affairs Outsourcing Market
3.7.1 COVID-19 Impact On Clinical Trial Services Market
3.7.2 COVID-19 Impact On Regulatory Services Market
3.7.3 COVID-19 Impact On Key Market Players
3.7.4 Post COVID-19 Impact On The Market
3.8 Top Regional Player Analysis
3.9 Total Number Of Clinical Trials, by Phase & Region (2021-23):
3.10 Average Cost/Price Of Clinical Trials
3.10.1 Average Cost/Price Of Clinical Trials By Phase
3.10.2 Average Cost/Price Of Clinical Trials By Therapeutic Areas
3.10.3 Average Cost/Price Of Clinical Trials By Major Countries/Region
3.11 Cost Breakdown Analysis For CRO's
3.11.1 Percent Of Outsourced Vs In-House Clinical Trial Categories
3.12 Regulatory Affairs: Industry Analysis Tools
3.12.1 PORTER’S Analysis
3.12.2 PESTEL Analysis
Chapter 4 Regulatory Affairs Outsourcing Market: Service Estimates & Trend Analysis
4.1 Service Movement Analysis & Market Share, 2023 & 2030
4.2 Regulatory Affairs Outsourcing Market Estimates & Forecast, By Service (USD Million)
4.2.1 Regulatory Consulting
4.2.1.1 Regulatory Consulting Market, 2018 - 2030 (USD Million)
4.2.1.2 Strategy & Development Planning
4.2.1.2.1 Strategy & Development Planning Market, 2018 - 2030 (USD Million)
4.2.1.3 QA Consulting
4.2.1.3.1 QA Consulting Market, 2018 - 2030 (USD Million)
4.2.1.4 Others
4.2.1.4.1 Others Market, 2018 - 2030 (USD Million)
4.2.2 Legal Representation
4.2.2.1 Legal Representation Market, 2018 - 2030 (USD Million)
4.2.3 Regulatory Writing & Publishing
4.2.3.1 Regulatory Writing & Publishing Market, 2018 - 2030 (USD Million)
4.2.4 Product Registration & Clinical Trial Applications
4.2.4.1 Product registration & clinical trial applications Market, 2018 - 2030 (USD Million)
4.2.5 Regulatory Submissions
4.2.5.1 Regulatory Submissions Market, 2018 - 2030 (USD Million)
4.2.6 Regulatory Operations
4.2.6.1 Regulatory Operations Market, 2018 - 2030 (USD Million)
4.2.7 Other Services
4.2.7.1 Other services Market, 2018 - 2030 (USD Million)
Chapter 5 Regulatory Affairs Outsourcing Market: Category Estimates & Trend Analysis
5.1 Category Movement Analysis & Market Share, 2023 & 2030
5.2 Regulatory Affairs Outsourcing Market Estimates & Forecast, By Category (USD Million)
5.3 Pharmaceuticals
5.3.1 Pharmaceuticals Regulatory Affairs Outsourcing Market, By Regulatory Consulting, 2018 - 2030 (USD Million)
5.3.1.1 Pharmaceutical Regulatory Affairs Outsourcing Market By Strategy & Development Planning, 2018 - 2030 (USD Million)
5.3.1.2 Pharmaceutical Regulatory Affairs Outsourcing Market By QA Consulting, 2018 - 2030 (USD Million)
5.3.1.3 Pharmaceutical Regulatory Affairs Outsourcing Market By Others,2018 - 2030 (USD Million)
5.3.2 Pharmaceuticals Regulatory Affairs Outsourcing Market, By Legal Representation, 2018 - 2030 (USD Million)
5.3.3 Pharmaceuticals Regulatory Affairs Outsourcing Market, By Regulatory Writing & Publishing, 2018 - 2030 (USD Million)
5.3.4 Pharmaceuticals Regulatory Affairs Outsourcing Market, By Product Registration & Clinical Trial Applications, 2018 - 2030 (USD Million)
5.3.5 Pharmaceuticals Regulatory Affairs Outsourcing Market, By Regulatory Submissions, 2018 - 2030 (USD Million)
5.3.6 Pharmaceuticals Regulatory Affairs Outsourcing Market, By Regulatory Operations, 2018 - 2030 (USD Million)
5.3.7 Pharmaceuticals Regulatory Affairs Outsourcing Market, By Other Services, 2018 - 2030 (USD Million)
5.4 Medical Devices
5.4.1 Medical Devices Regulatory Affairs Outsourcing Market By Regulatory Consulting, 2018 - 2030 (USD Million)
5.4.1.1 Medical Devices Regulatory Affairs Outsourcing Market By Strategy & Development Planning, 2018 - 2030 (USD Million)
5.4.1.2 Medical Devices Regulatory Affairs Outsourcing Market By QA Consulting, 2018 - 2030 (USD Million)
5.4.1.3 Medical Devices Regulatory Affairs Outsourcing Market By Others, 2018 - 2030 (USD Million)
5.4.2 Medical Devices Regulatory Affairs Outsourcing Market By Legal Representation, 2018 - 2030 (USD Million)
5.4.3 Medical Devices Regulatory Affairs Outsourcing Market By Regulatory Writing And Publishing, 2018 - 2030 (USD Million)
5.4.4 Medical Devices Regulatory Affairs Outsourcing Market By Product Registration & Clinical Trial Applications, 2018 - 2030 (USD Million)
5.4.5 Medical Devices Regulatory Affairs Outsourcing Market By Regulatory Submissions, 2018 - 2030 (USD Million)
5.4.6 Medical Devices Regulatory Affairs Outsourcing Market By Regulatory Operations, 2018 - 2030 (USD Million)
5.4.7 Medical Devices Regulatory Affairs Outsourcing Market By Other Services, 2018 - 20302018 - 20302018 - 2030 (USD Million)
Chapter 6 Regulatory Affairs Outsourcing Market: Company Size Estimates & Trend Analysis
6.1 Company Size Movement Analysis & Market Share, 2022 & 2030
6.2 Regulatory Affairs Outsourcing Market Estimates & Forecast, By Company Size (USD Million)
6.2.1 Small Companies
6.2.1.1 Small Companies Market, 2018 - 2030 (USD Million)
6.2.2 Medium Companies
6.2.2.1 Medium Companies Market, 2018 - 2030 (USD Million)
6.2.3 Large Companies
6.2.3.1 Large Companies Market, 2018 - 2030 (USD Million)
Chapter 7 Regulatory Affairs Outsourcing Market: Indication Estimates & Trend Analysis
7.1 Indication Movement Analysis & Market Share, 2022 & 2030
7.2 Regulatory Affairs Outsourcing Market Estimates & Forecast, By Indication (USD Million)
7.2.1 Oncology
7.2.1.1 Oncology Market, 2018 - 2030 (USD Million)
7.2.2 Neurology
7.2.2.1 Neurology Market, 2018 - 2030 (USD Million)
7.2.3 Cardiology
7.2.3.1 Cardiology Market, 2018 - 2030 (USD Million)
7.2.4 Immunology
7.2.4.1 Immunology Market, 2018 - 2030 (USD Million)
7.2.5 Other Indications
7.2.5.1 Other Indications Market, 2018 - 2030 (USD Million)
Chapter 8 Regulatory Affairs Outsourcing Market: Product Stage Estimates & Trend Analysis
8.1 Product Stage Movement Analysis & Market Share, 2022 & 2030
8.2 Regulatory Affairs Outsourcing Market Estimates & Forecast, By Product Stage (USD Million)
8.2.1 Preclinical
8.2.1.1 Preclinical Market, 2018 - 2030 (USD Million)
8.2.2 Clinical
8.2.2.1 Clinical Market, 2018 - 2030 (USD Million)
8.2.3 Premarket Approval (PMA)
8.2.3.1 Premarket Approval (PMA) Market, 2018 - 2030 (USD Million)
Chapter 9 Regulatory Affairs Outsourcing Market: End-Use Estimates & Trend Analysis
9.1 End-Use Movement Analysis & Market Share, 2022 & 2030
9.2 Regulatory Affairs Outsourcing Market Estimates & Forecast, By End-Use (USD Million)
9.2.1 Medical Device Companies
9.2.1.1 Medical Device Companies Market, 2018 - 2030 (USD Million)
9.2.2 Pharmaceutical Companies
9.2.2.1 Pharmaceutical Companies Market, 2018 - 2030 (USD Million)
9.2.3 Biotechnology Companies
9.2.3.1 Biotechnology Companies Market, 2018 - 2030 (USD Million)
Chapter 10 Regulatory Affairs Outsourcing Market: Regional Estimates and Trend Analysis
10.1 Regulatory Affairs Outsourcing Market: Regional Outlook
10.2 North America
10.2.1 North America Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.2.2 U.S.
10.2.2.1 Key Country Dynamics
10.2.2.2 Regulatory Landscape/Reimbursement Scenario
10.2.2.3 Competitive Insights
10.2.2.4 U.S. Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.2.3 Canada
10.2.3.1 Key Country Dynamics
10.2.3.2 Regulatory Landscape/Reimbursement Scenario
10.2.3.3 Competitive Insights
10.2.3.4 Canada Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3 Europe
10.3.1 Europe Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.2 UK
10.3.2.1 Key Country Dynamics
10.3.2.2 Regulatory Landscape/Reimbursement Scenario
10.3.2.3 Competitive Insights
10.3.2.4 UK Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.3 Germany
10.3.3.1 Key Country Dynamics
10.3.3.2 Regulatory Landscape/Reimbursement Scenario
10.3.3.3 Competitive Insights
10.3.3.4 Germany Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.4 France
10.3.4.1 Key Country Dynamics
10.3.4.2 Regulatory Landscape/Reimbursement Scenario
10.3.4.3 Competitive Insights
10.3.4.4 France Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.5 Italy
10.3.5.1 Key Country Dynamics
10.3.5.2 Regulatory Landscape/Reimbursement Scenario
10.3.5.3 Competitive Insights
10.3.5.4 Italy Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.6 Spain
10.3.6.1 Key Country Dynamics
10.3.6.2 Regulatory Landscape/Reimbursement Scenario
10.3.6.3 Competitive Insights
10.3.6.4 Spain Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.7 Russia
10.3.7.1 Key Country Dynamics
10.3.7.2 Regulatory Landscape/Reimbursement Scenario
10.3.7.3 Competitive Insights
10.3.7.4 Russia Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.8 Sweden
10.3.8.1 Key Country Dynamics
10.3.8.2 Regulatory Landscape/Reimbursement Scenario
10.3.8.3 Competitive Insights
10.3.8.4 Sweden Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.9 Turkey
10.3.9.1 Key Country Dynamics
10.3.9.2 Regulatory Landscape/Reimbursement Scenario
10.3.9.3 Competitive Insights
10.3.9.4 Turkey Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.10 Netherlands
10.3.10.1 Key Country Dynamics
10.3.10.2 Regulatory Landscape/Reimbursement Scenario
10.3.10.3 Competitive Insights
10.3.10.4 Netherlands Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.11 Switzerland
10.3.11.1 Key Country Dynamics
10.3.11.2 Regulatory Landscape/Reimbursement Scenario
10.3.11.3 Competitive Insights
10.3.11.4 Switzerland Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.3.12 Europe CIS Countries
10.3.12.1 Key Country Dynamics
10.3.12.2 Regulatory Landscape/Reimbursement Scenario
10.3.12.3 Competitive Insights
10.3.12.4 Europe CIS Countries Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4 Asia Pacific
10.4.1 Asia Pacific Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.2 Japan
10.4.2.1 Key Country Dynamics
10.4.2.2 Regulatory Landscape/Reimbursement Scenario
10.4.2.3 Competitive Insights
10.4.2.4 Japan Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.3 China
10.4.3.1 Key Country Dynamics
10.4.3.2 Regulatory Landscape/Reimbursement Scenario
10.4.3.3 Competitive Insights
10.4.3.4 China Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.4 India
10.4.4.1 Key Country Dynamics
10.4.4.2 Regulatory Landscape/Reimbursement Scenario
10.4.4.3 Competitive Insights
10.4.4.4 India Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.5 Australia
10.4.5.1 Key Country Dynamics
10.4.5.2 Regulatory Landscape/Reimbursement Scenario
10.4.5.3 Competitive Insights
10.4.5.4 Australia Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.6 South Korea
10.4.6.1 Key Country Dynamics
10.4.6.2 Regulatory Landscape/Reimbursement Scenario
10.4.6.3 Competitive Insights
10.4.6.4 South Korea Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.7 Thailand
10.4.7.1 Key Country Dynamics
10.4.7.2 Regulatory Landscape/Reimbursement Scenario
10.4.7.3 Competitive Insights
10.4.7.4 Thailand Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.8 Indonesia
10.4.8.1 Key Country Dynamics
10.4.8.2 Regulatory Landscape/Reimbursement Scenario
10.4.8.3 Competitive Insights
10.4.8.4 Indonesia Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.9 Malaysia
10.4.9.1 Key Country Dynamics
10.4.9.2 Regulatory Landscape/Reimbursement Scenario
10.4.9.3 Competitive Insights
10.4.9.4 Malaysia Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.10 Singapore
10.4.10.1 Key Country Dynamics
10.4.10.2 Regulatory Landscape/Reimbursement Scenario
10.4.10.3 Competitive Insights
10.4.10.4 Singapore Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.11 Taiwan
10.4.11.1 Key Country Dynamics
10.4.11.2 Regulatory Landscape/Reimbursement Scenario
10.4.11.3 Competitive Insights
10.4.11.4 Taiwan Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.4.12 CIS Countries
10.4.12.1 Key Country Dynamics
10.4.12.2 Regulatory Landscape/Reimbursement Scenario
10.4.12.3 Competitive Insights
10.4.12.4 Asia Pacific CIS Countries Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.5 Latin America
10.5.1 Latin America Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.5.2 Brazil
10.5.1.1 Key Country Dynamics
10.5.1.2 Regulatory Landscape/Reimbursement Scenario
10.5.1.3 Competitive Insights
10.5.1.4 Brazil Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.5.3 Mexico
10.5.3.1 Key Country Dynamics
10.5.3.2 Regulatory Landscape/Reimbursement Scenario
10.5.3.3 Competitive Insights
10.5.3.4 Mexico Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.5.4 Argentina
10.5.4.1 Key Country Dynamics
10.5.4.2 Regulatory Landscape/Reimbursement Scenario
10.5.4.3 Competitive Insights
10.5.4.4 Argentina Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.5.5 Colombia
10.5.5.1 Key Country Dynamics
10.5.5.2 Regulatory Landscape/Reimbursement Scenario
10.5.5.3 Competitive Insights
10.5.5.4 Colombia Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.5.6 Chile
10.5.6.1 Key Country Dynamics
10.5.6.2 Regulatory Landscape/Reimbursement Scenario
10.5.6.3 Competitive Insights
10.5.6.4 Chile Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.6 Middle East & Africa
10.6.1 Middle East & Africa Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.6.2 South Africa
10.6.2.1 Key Country Dynamics
10.6.2.2 Regulatory Landscape/Reimbursement Scenario
10.6.2.3 Competitive Insights
10.6.2.4 South Africa Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.6.3 Saudi Arabia
10.6.3.1 Key Country Dynamics
10.6.3.2 Regulatory Landscape/Reimbursement Scenario
10.6.3.3 Competitive Insights
10.6.3.4 Saudi Arabia Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.6.4 UAE
10.6.4.1 Key Country Dynamics
10.6.4.2 Regulatory Landscape/Reimbursement Scenario
10.6.4.3 Competitive Insights
10.6.4.4 UAE Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.6.5 Egypt
10.6.5.1 Key Country Dynamics
10.6.5.2 Regulatory Landscape/Reimbursement Scenario
10.6.5.3 Competitive Insights
10.6.5.4 Egypt Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.6.6 Israel
10.6.6.1 Key Country Dynamics
10.6.6.2 Regulatory Landscape/Reimbursement Scenario
10.6.6.3 Competitive Insights
10.6.6.4 Israel Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
10.6.7 Kuwait
10.6.7.1 Key Country Dynamics
10.6.7.2 Regulatory Landscape/Reimbursement Scenario
10.6.7.3 Competitive Insights
10.6.7.4 Kuwait Regulatory Affairs Outsourcing Market Estimates And Forecasts, 2018 - 2030 (USD Million)
Chapter 11 Competitive Landscape
11.1 Recent Developments & Impact Analysis by Key Market Participants
11.2 Company Categorization
11.3 Company Market Share Analysis
11.4 Company Heat Map Analysis
11.5. Strategy Mapping
11.5.1 Expansions
11.5.2 Merger & Acquisition
11.5.3 Partnerships & Collaborations
11.5.4 Service Launches
11.5.5 Research And Development
11.6. Company Profiles
11.6.1 Accell Clinical Research, LLC
11.6.1.1 Participant’s Overview
11.6.1.2 Financial Performance
11.6.1.3 Service Benchmarking
11.6.1.4 Strategic Initiatives
11.6.2 Genpact
11.6.2.1 Participant’s Overview
11.6.2.2 Financial Performance
11.6.2.3 Service Benchmarking
11.6.2.4 Strategic Initiatives
11.6.3 CRITERIUM, INC
11.6.3.1 Participant’s Overview
11.6.3.2 Financial Performance
11.6.3.3 Service Benchmarking
11.6.3.4 Strategic Initiatives
11.6.4 Promedica International
11.6.4.1 Participant’s Overview
11.6.4.2 Financial Performance
11.6.4.3 Service Benchmarking
11.6.4.4 Strategic Initiatives
11.6.5 WuXi AppTec
11.6.5.1 Participant’s Overview
11.6.5.2 Financial Performance
11.6.5.3 Service Benchmarking
11.6.5.4 Strategic Initiatives
11.6.6 Medpace
11.6.6.1 Participant’s Overview
11.6.6.2 Financial Performance
11.6.6.3 Service Benchmarking
11.6.6.4 Strategic Initiatives
11.6.7 Charles River Laboratories
11.6.7.1 Participant’s Overview
11.6.7.2 Financial Performance
11.6.7.3 Service Benchmarking
11.6.7.4 Strategic Initiatives
11.6.8 ICN plc
11.6.8.1 Participant’s Overview
11.6.8.2 Financial Performance
11.6.8.3 Service Benchmarking
11.6.8.4 Strategic Initiatives
11.6.9 Labcorp Drug Development
11.6.9.1 Participant’s Overview
11.6.9.2 Financial Performance
11.6.9.3 Service Benchmarking
11.6.9.4 Strategic Initiatives
11.6.10 Parexel International Corporation
11.6.10.1 Participant’s Overview
11.6.10.2 Financial Performance
11.6.10.3 Service Benchmarking
11.6.10.4 Strategic Initiatives
11.6.11 Freyr
11.6.11.1 Participant’s Overview
11.6.11.2 Financial Performance
11.6.11.3 Service Benchmarking
11.6.11.4 Strategic Initiatives
11.6.12 PHARMALEX GMBH
11.6.12.1 Participant’s Overview
11.6.12.2 Financial Performance
11.6.12.3 Service Benchmarking
11.6.12.4 Strategic Initiatives
11.6.13 NDA Group AB
11.6.13.1 Participant’s Overview
11.6.13.2 Financial Performance
11.6.13.3 Service Benchmarking
11.6.13.4 Strategic Initiatives
11.6.14 Pharmexon
11.6.14.1 Participant’s Overview
11.6.14.2 Financial Performance
11.6.14.3 Service Benchmarking
11.6.14.4 Strategic Initiatives
11.6.15 Qvigilance
11.6.15.1 Participant’s Overview
11.6.15.2 Financial Performance
11.6.15.3 Service Benchmarking
11.6.15.4 Strategic Initiatives
11.6.16 BlueReg
11.6.16.1 Participant’s Overview
11.6.16.2 Financial Performance
11.6.16.3 Service Benchmarking
11.6.16.4 Strategic Initiatives
11.6.17 Cambridge Regulatory Services
11.6.17.1 Participant’s Overview
11.6.17.2 Financial Performance
11.6.17.3 Service Benchmarking
11.6.17.4 Strategic Initiatives
11.6.18 APCER Life Sciences, Inc.
11.6.18.1 Participant’s Overview
11.6.18.2 Financial Performance
11.6.18.3 Service Benchmarking
11.6.18.4 Strategic Initiatives
11.6.19 Real Regulatory Ltd
11.6.19.1 Participant’s Overview
11.6.19.2 Financial Performance
11.6.19.3 Service Benchmarking
11.6.19.4 Strategic Initiatives
11.6.20 VCLS
11.6.20.1 Participant’s Overview
11.6.20.2 Financial Performance
11.6.20.3 Service Benchmarking
11.6.20.4 Strategic Initiatives
11.6.21 PrimeVigilance
11.6.21.1 Participant’s Overview
11.6.21.2 Financial Performance
11.6.21.3 Service Benchmarking
11.6.21.4 Strategic Initiatives
11.6.22 ProPharma Group MIS Limited
11.6.22.1 Participant’s Overview
11.6.22.2 Financial Performance
11.6.22.3 Service Benchmarking
11.6.22.4 Strategic Initiatives
11.6.23 Regulatory Pharma Net srl
11.6.23.1 Participant’s Overview
11.6.23.2 Financial Performance
11.6.23.3 Service Benchmarking
11.6.23.4 Strategic Initiatives
11.6.24 ZEINCRO
11.6.24.1 Participant’s Overview
11.6.24.2 Financial Performance
11.6.24.3 Service Benchmarking
11.6.24.4 Strategic Initiatives
11.6.25 BioMapas
11.6.25.1 Participant’s Overview
11.6.25.2 Financial Performance
11.6.25.3 Service Benchmarking
11.6.25.4 Strategic Initiatives
11.6.26 REGENOLD GMBH
11.6.26.1 Participant’s Overview
11.6.26.2 Financial Performance
11.6.26.3 Service Benchmarking
11.6.26.4 Strategic Initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Abbreviations
Table 3 Global Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 4 Global Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 5 Global Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 6 Global Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 7 Global Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 8 Global Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 9 Global Regulatory Affairs Outsourcing Market, by Region, 2018 - 2030 (USD Million)
Table 10 North America Regulatory Affairs Outsourcing Market, by Country, 2018 - 2030 (USD Million)
Table 11 North America Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 12 North America Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 13 North America Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 14 North America Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 15 North America Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 16 North America Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 17 U.S. Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 18 U.S. Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 19 U.S. Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 20 U.S. Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 21 U.S. Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 22 U.S. Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 23 Canada Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 24 Canada Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 25 Canada Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 26 Canada Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 27 Canada Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 28 Canada Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 29 Europe Regulatory Affairs Outsourcing Market, by Country, 2018 - 2030 (USD Million)
Table 30 Europe Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 31 Europe Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 32 Europe Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 33 Europe Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 34 Europe Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 35 Europe Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 36 Germany Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 37 Germany Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 38 Germany Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 39 Germany Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 40 Germany Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 41 Germany Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 42 UK Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 43 UK Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 44 UK Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 45 UK Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 46 UK Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 47 UK Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 48 France Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 49 France Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 50 France Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 51 France Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 52 France Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 53 France Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 54 Italy Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 55 Italy Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 56 Italy Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 57 Italy Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 58 Italy Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 59 Italy Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 60 Spain Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 61 Spain Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 62 Spain Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 63 Spain Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 64 Spain Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 65 Spain Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 66 Denmark Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 67 Denmark Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 68 Denmark Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 69 Denmark Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 70 Denmark Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 71 Denmark Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 72 Sweden Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 73 Sweden Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 74 Sweden Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 75 Sweden Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 76 Sweden Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 77 Sweden Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 78 Norway Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 79 Norway Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 80 Norway Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 81 Norway Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 82 Norway Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 83 Norway Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 84 Asia Pacific Regulatory Affairs Outsourcing Market, by Country, 2018 - 2030 (USD Million)
Table 85 Asia Pacific Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 86 Asia Pacific Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 87 Asia Pacific Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 88 Asia Pacific Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 89 Asia Pacific Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 90 Asia Pacific Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 91 China Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 92 China Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 93 China Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 94 China Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 95 China Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 96 China Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 97 Japan Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 98 Japan Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 99 Japan Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 100 Japan Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 101 Japan Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 102 Japan Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 103 India Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 104 India Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 105 India Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 106 India Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 107 India Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 108 India Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 109 South Korea Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 110 South Korea Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 111 South Korea Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 112 South Korea Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 113 South Korea Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 114 South Korea Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 115 Australia Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 116 Australia Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 117 Australia Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 118 Australia Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 119 Australia Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 120 Australia Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 121 Thailand Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 122 Thailand Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 123 Thailand Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 124 Thailand Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 125 Thailand Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 126 Thailand Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 127 Latin America Regulatory Affairs Outsourcing Market, by Country, 2018 - 2030 (USD Million)
Table 128 Latin America Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 129 Latin America Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 130 Latin America Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 131 Latin America Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 132 Latin America Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 133 Latin America Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 134 Brazil Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 135 Brazil Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 136 Brazil Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 137 Brazil Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 138 Brazil Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 139 Brazil Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 140 Mexico Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 141 Mexico Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 142 Mexico Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 143 Mexico Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 144 Mexico Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 145 Mexico Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 146 Argentina Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 147 Argentina Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 148 Argentina Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 149 Argentina Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 150 Argentina Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 151 Argentina Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 152 Middle East & Africa Regulatory Affairs Outsourcing Market, by Country, 2018 - 2030 (USD Million)
Table 153 Middle East & Africa Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 154 Middle East & Africa Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 155 Middle East & Africa Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 156 Middle East & Africa Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 157 Middle East & Africa Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 158 Middle East & Africa Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 159 South Africa Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 160 South Africa Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 161 South Africa Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 162 South Africa Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 163 South Africa Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 164 South Africa Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 165 Saudi Arabia Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 166 Saudi Arabia Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 167 Saudi Arabia Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 168 Saudi Arabia Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 169 Saudi Arabia Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 170 Saudi Arabia Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 171 UAE Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 172 UAE Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 173 UAE Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 174 UAE Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 175 UAE Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 176 UAE Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
Table 177 Kuwait Regulatory Affairs Outsourcing Market, by Service, 2018 - 2030 (USD Million)
Table 178 Kuwait Regulatory Affairs Outsourcing Market, by Category, 2018 - 2030 (USD Million)
Table 179 Kuwait Regulatory Affairs Outsourcing Market, by Company Size, 2018 - 2030 (USD Million)
Table 180 Kuwait Regulatory Affairs Outsourcing Market, by Indication, 2018 - 2030 (USD Million)
Table 181 Kuwait Regulatory Affairs Outsourcing Market, by Product Stage, 2018 - 2030 (USD Million)
Table 182 Kuwait Regulatory Affairs Outsourcing Market, by End-Use, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Information Procurement
Fig. 2 Primary Research Pattern
Fig. 3 Market Research Approaches
Fig. 4 Value Chain-Based Sizing & Forecasting
Fig. 5 Market Formulation & Validation
Fig. 6 Regulatory Affairs Outsourcing Market, Market Segmentation
Fig. 7 Market Driver Relevance Analysis (Current & Future Impact)
Fig. 8 Market Restraint Relevance Analysis (Current & Future Impact)
Fig. 9 SWOT Analysis, By Factor (Political & Legal, Economic and Technological)
Fig. 10 Porter’s Five Forces Analysis
Fig. 11 Regional Marketplace: Key Takeaways
Fig. 12 Global Regulatory Affairs Outsourcing Market, for Regulatory consulting, 2018 - 2030 (USD Million)
Fig. 13 Global Regulatory Affairs Outsourcing Market, for Strategy & Development Planning, 2018 - 2030 (USD Million)
Fig. 14 Global Regulatory Affairs Outsourcing Market, for QA Consulting, 2018 - 2030 (USD Million)
Fig. 15 Global Regulatory Affairs Outsourcing Market, for Agent Services, 2018 - 2030 (USD Million)
Fig. 16 Global Regulatory Affairs Outsourcing Market, for Others, 2018 - 2030 (USD Million)
Fig. 17 Global Regulatory Affairs Outsourcing Market, for Legal representation, 2018 - 2030 (USD Million)
Fig. 18 Global Regulatory Affairs Outsourcing Market, for Regulatory writing & publishing, 2018 - 2030 (USD Million)
Fig. 19 Global Regulatory Affairs Outsourcing Market, for Onsite Monitoring, 2018 - 2030 (USD Million)
Fig. 20 Global Regulatory Affairs Outsourcing Market, for Product registration & clinical trial applications, 2018 - 2030 (USD Million)
Fig. 21 Global Regulatory Affairs Outsourcing Market, for Project Management Services, 2018 - 2030 (USD Million)
Fig. 22 Global Regulatory Affairs Outsourcing Market, for Others, 2018 - 2030 (USD Million)
Fig. 23 Global Regulatory Affairs Outsourcing Market, for Regulatory Submissions, 2018 - 2030 (USD Million)
Fig. 24 Global Regulatory Affairs Outsourcing Market, for Regulatory Operations, 2018 - 2030 (USD Million)
Fig. 25 Global Regulatory Affairs Outsourcing Market, for other services, 2018 - 2030 (USD Million)
Fig. 26 Global Regulatory Affairs Outsourcing Market, for Drugs, 2018 - 2030 (USD Million)
Fig. 27 Global Regulatory Affairs Outsourcing Market, for Innovators, 2018 - 2030 (USD Million)
Fig. 28 Global Regulatory Affairs Outsourcing Market, for Generics, 2018 - 2030 (USD Million)
Fig. 29 Global Regulatory Affairs Outsourcing Market, for Biologics, 2018 - 2030 (USD Million)
Fig. 30 Global Regulatory Affairs Outsourcing Market, for Biotech, 2018 - 2030 (USD Million)
Fig. 31 Global Regulatory Affairs Outsourcing Market, for ATMP, 2018 - 2030 (USD Million)
Fig. 32 Global Regulatory Affairs Outsourcing Market, for Biosimilars, 2018 - 2030 (USD Million)
Fig. 33 Global Regulatory Affairs Outsourcing Market, for Medical Devices, 2018 - 2030 (USD Million)
Fig. 34 Global Regulatory Affairs Outsourcing Market, for Diagnostics, 2018 - 2030 (USD Million)
Fig. 35 Global Regulatory Affairs Outsourcing Market, for Therapeutics, 2018 - 2030 (USD Million)
Fig. 36 Global Regulatory Affairs Outsourcing Market, for Small, 2018 - 2030 (USD Million)
Fig. 37 Global Regulatory Affairs Outsourcing Market, for Small Sized Biotech/biopharmaceutical Companies, 2018 - 2030 (USD Million)
Fig. 38 Global Regulatory Affairs Outsourcing Market, for Small Sized Pharmaceutical Companies, 2018 - 2030 (USD Million)
Fig. 39 Global Regulatory Affairs Outsourcing Market, for Small Sized Medical Device Companies, 2018 - 2030 (USD Million)
Fig. 40 Global Regulatory Affairs Outsourcing Market, for Medium, 2018 - 2030 (USD Million)
Fig. 41 Global Regulatory Affairs Outsourcing Market, for Medium Sized Biotech/biopharmaceutical Companies, 2018 - 2030 (USD Million)
Fig. 42 Global Regulatory Affairs Outsourcing Market, for Medium Sized Pharmaceutical Companies, 2018 - 2030 (USD Million)
Fig. 43 Global Regulatory Affairs Outsourcing Market, for Medium Sized Medical Device Companies, 2018 - 2030 (USD Million)
Fig. 44 Global Regulatory Affairs Outsourcing Market, for Large, 2018 - 2030 (USD Million)
Fig. 45 Global Regulatory Affairs Outsourcing Market, for Large Sized Biotech/biopharmaceutical Companies, 2018 - 2030 (USD Million)
Fig. 46 Global Regulatory Affairs Outsourcing Market, for Large Sized Pharmaceutical Companies, 2018 - 2030 (USD Million)
Fig. 47 Global Regulatory Affairs Outsourcing Market, for Large Sized Medical Device Companies, 2018 - 2030 (USD Million)
Fig. 48 Global Regulatory Affairs Outsourcing Market, for Oncology, 2018 - 2030 (USD Million)
Fig. 49 Global Regulatory Affairs Outsourcing Market, for Neurology, 2018 - 2030 (USD Million)
Fig. 50 Global Regulatory Affairs Outsourcing Market, for Cardiology, 2018 - 2030 (USD Million)
Fig. 51 Global Regulatory Affairs Outsourcing Market, for immunology, 2018 - 2030 (USD Million)
Fig. 52 Global Regulatory Affairs Outsourcing Market, for Others, 2018 - 2030 (USD Million)
Fig. 53 Global Regulatory Affairs Outsourcing Market, for Preclinical, 2018 - 2030 (USD Million)
Fig. 54 Global Regulatory Affairs Outsourcing Market, for Clinical studies, 2018 - 2030 (USD Million)
Fig. 55 Global Regulatory Affairs Outsourcing Market, for PMA, 2018 - 2030 (USD Million)
Fig. 56 Global Regulatory Affairs Outsourcing Market, for Biotechnology Companies, 2018 - 2030 (USD Million)
Fig. 57 Global Regulatory Affairs Outsourcing Market, for Pharmaceutical Companies, 2018 - 2030 (USD Million)
Fig. 58 Global Regulatory Affairs Outsourcing Market, for Medical Device Companies, 2018 - 2030 (USD Million)
Fig. 59 Regional Outlook, 2023 & 2030
Fig. 60 North America Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 61 U.S. Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 62 Canada Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 63 Europe Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 64 Germany Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 65 UK Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 66 France Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 67 Italy Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 68 Spain Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 69 Denmark Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 70 Sweden Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 71 Norway Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 72 Turkey Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 73 Netherlands Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 74 Switzerland Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 75 CIS Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 76 Asia Pacific Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 77 Japan Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 78 China Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 79 India Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 80 Australia Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 81 South Korea Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 82 Thailand Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 83 Indonesia Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 84 Malaysia Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 85 Singapore Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 86 Taiwan Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 87 CIS Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 88 Latin America Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 89 Brazil Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 90 Mexico Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 91 Argentina Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 92 Colombia Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 93 Chile Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 94 Middle East and Africa Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 95 South Africa Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 96 Saudi Arabia Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 97 UAE Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 98 Kuwait Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 99 Egypt Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)
Fig. 100 Israel Regulatory Affairs Outsourcing Market, 2018 - 2030 (USD Million)What questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Regulatory Affairs Outsourcing Market: Regional Outlook (Revenue, USD Million, 2018- 2030)
- North America
- Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- U.S.
- U.S. Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- U.S. Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- U.S. Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- U.S. Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- U.S. Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- U.S. Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- U.S. Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Canada
- Canada Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Canada Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Canada Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Canada Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Canada Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Canada Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Canada Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Europe
- Europe Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Regulatory Consulting
- Europe Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Europe Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Europe Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Europe Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- UK
- UK Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- UK Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- UK Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- UK Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- UK Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- UK Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- UK Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Germany
- Germany Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Germany Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Germany Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Germany Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Germany Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Germany Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Germany Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- France
- France Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- France Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- France Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- France Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- France Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- France Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- France Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Italy
- Italy Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Italy Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Italy Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Italy Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Italy Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Italy Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Italy Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Spain
- Spain Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Spain Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Spain Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Spain Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Spain Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Spain Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Spain Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Russia
- Russia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Russia Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Russia Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Russia Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Russia Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Russia Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Russia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Denmark
- Denmark Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Denmark Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Denmark Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Denmark Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Denmark Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Denmark Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Denmark Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Sweden
- Sweden Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Sweden Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Sweden Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Sweden Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Sweden Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Sweden Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Sweden Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Norway
- Norway Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Norway Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Norway Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Norway Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Norway Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Norway Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Norway Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Turkey
- Turkey Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Turkey Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Turkey Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Turkey Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Turkey Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Turkey Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Turkey Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Netherlands
- Netherlands Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Netherlands Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Netherlands Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Netherlands Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Netherlands Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Netherlands Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Netherlands Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Switzerland
- Switzerland Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Switzerland Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Switzerland Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Switzerland Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Switzerland Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Switzerland Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Switzerland Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- CIS
- CIS Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- CIS Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- CIS Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- CIS Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- CIS Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- CIS Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- CIS Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Europe Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Asia Pacific
- Asia Pacific Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Asia Pacific Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Asia Pacific Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Asia Pacific Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Asia Pacific Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Asia Pacific Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Japan
- Japan Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Japan Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Japan Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Japan Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Japan Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- China
- China Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- China Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- China Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- China Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- China Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- China Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- China Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- India
- India Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- India Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- India Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- India Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- India Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- India Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- India Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- South Korea
- South Korea Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- South Korea Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- South Korea Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- South Korea Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- South Korea Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- South Korea Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- South Korea Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Australia
- Australia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Australia Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Australia Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Australia Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Australia Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Australia Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Australia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Thailand
- Thailand Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Thailand Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Thailand Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Thailand Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Thailand Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Thailand Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Thailand Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Singapore
- Singapore Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Singapore Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Singapore Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Singapore Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Singapore Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Singapore Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Singapore Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Indonesia
- Indonesia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Indonesia Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Indonesia Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Indonesia Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Indonesia Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Indonesia Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Indonesia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Malaysia
- Malaysia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Malaysia Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Malaysia Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Malaysia Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Malaysia Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Malaysia Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Malaysia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Taiwan
- Taiwan Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Taiwan Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Taiwan Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Taiwan Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Taiwan Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Taiwan Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Taiwan Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- CIS
- CIS Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- CIS Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- CIS Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- CIS Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- CIS Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- CIS Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- CIS Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Latin America
- Latin America Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Latin America Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Latin America Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Latin America Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Latin America Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Latin America Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Brazil
- Brazil Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Brazil Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Brazil Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Brazil Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Brazil Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Brazil Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Brazil Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Mexico
- Mexico Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Mexico Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Mexico Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Mexico Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Mexico Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Mexico Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Mexico Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Argentina
- Argentina Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Argentina Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Argentina Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Argentina Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Argentina Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Argentina Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Argentina Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Colombia
- Colombia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Colombia Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Colombia Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Colombia Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Colombia Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Colombia Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Colombia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Chile
- Chile Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Chile Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Chile Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Chile Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Chile Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Chile Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Chile Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Latin America Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- MEA
- MEA Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- MEA Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- MEA Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- MEA Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- MEA Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- MEA Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- South Africa
- South Africa Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- South Africa Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- South Africa Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- South Africa Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- South Africa Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- South Africa Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- South Africa Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Saudi Arabia
- Saudi Arabia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Saudi Arabia Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Saudi Arabia Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Saudi Arabia Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Saudi Arabia Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Saudi Arabia Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Saudi Arabia Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- UAE
- UAE Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- UAE Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- UAE Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- UAE Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- UAE Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- UAE Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- UAE Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Egypt
- Egypt Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Egypt Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Egypt Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Egypt Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Egypt Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Egypt Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Egypt Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Israel
- Israel Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Israel Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Israel Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Israel Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Israel Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Israel Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Israel Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Kuwait
- Kuwait Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submission
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Kuwait Regulatory Affairs Outsourcing Company Size Outlook (Revenue, USD Million, 2018 - 2030)
- Small
- Medium
- Large
- Kuwait Regulatory Affairs Outsourcing Category Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Regulatory Consulting
- Strategy & Development Planning
- QA consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Medical Device
- Regulatory Consulting
- Strategy & Development Planning
- QA Consulting
- Others
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Applications
- Regulatory Submissions
- Regulatory Operations
- Other Services
- Regulatory Consulting
- Pharmaceuticals
- Kuwait Regulatory Affairs Outsourcing Indication Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Kuwait Regulatory Affairs Outsourcing Product Stage Outlook (Revenue, USD Million, 2018 - 2030)
- Preclinical
- Clinical
- PMA (Post Market Authorization)
- Kuwait Regulatory Affairs Outsourcing End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical Companies
- Biotechnology Companies
- Kuwait Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- MEA Regulatory Affairs Outsourcing Service Outlook (Revenue, USD Million, 2018 - 2030)
- North America
Regulatory Affairs Outsourcing Market Dynamics
Driver: Changing Regulatory Landscape
Life sciences companies have to deal with continuous changes in regulatory requirements, which can differ based on business activities and geographies. Noncompliance with the changing regulatory requirement can result in penalties and delays, which may lead to loss of revenue. According to a survey sponsored by GenPact, 72.0% of executives from the life sciences industry consider regulatory compliance as one of the top three challenges faced by life sciences companies. Regulatory departments often face the burden of handling multiple tasks simultaneously and have to ensure compliance with stringent regulatory standards at all times. Increase in efforts by companies to expand their geographical reach and gain rapid approvals in global markets is expected to further contribute to the adoption of outsourcing models for regulatory services. Regulatory approval procedures are becoming increasingly stringent and market players aim to receive product approvals in the first attempt to gain market share.
Driver: Entry of Companies in the Global Market
Globalization of biopharmaceuticals and medical device companies is expected to be one of the major factors driving the market growth. Emerging markets from Latin America, Asia Pacific, and MEA offer low costs-product development & manufacturing costs, tax benefits, and availability of skilled labor at relatively low costs with supportive regulations. The abovementioned factors have made the regional markets attractive prospects in terms of outsourcing and expansion for biopharmaceutical & medical device companies, thus, stimulating the demand for regulatory services. For instance, in April 2018, B. Braun opened 5 medical production facilities in Penang, Malaysia. In June 2019, Flex Ltd. announced the opening of its new manufacturing facility in Tamil Nadu, India. Such initiatives are anticipated to fuel the demand for regulatory services in the countries, especially for GMP and product development
Restraint: Risk Associated with Data Security
According to an article published in Fierce Healthcare in 2019, data breaches in healthcare industry had nearly tripled over the previous year. According to IBM, the average cost of a data breach in healthcare is nearly USD 7.13 million in 2021, indicating an increase of nearly 10% from 2019. The increase in data breaches, coupled with availability of several electronic/digital solutions, such as eCTD submissions, digital IND safety reporting, and other digital documentation, is anticipated to increase the risk the cyber-attacks/data breaches in the regulatory affairs outsourcing market. The healthcare industry is characterized by a large number of collaborations, geographic expansions, and mergers & acquisitions. Mergers and acquisitions can create vulnerabilities in an organization, making it prone to cyberattacks. They involve the exchange of large volumes of vital information on legal contracts, terms of agreement, patient details, and provider & services information of all involved parties, thus increasing the risk of cyber-attacks.
What Does This Report Include?
This section will provide insights into the contents included in this regulatory affairs outsourcing market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Regulatory affairs outsourcing market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Regulatory affairs outsourcing market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the regulatory affairs outsourcing market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for regulatory affairs outsourcing market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of regulatory affairs outsourcing market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Regulatory Affairs Outsourcing Market Categorization:
The regulatory affairs outsourcing market was categorized into seven segments, namely services (Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Applications, Regulatory Submission, Regulatory Operations), company size (Small, Medium, Large), category (Pharmaceuticals, Medical Device), indication (Oncology, Neurology, Cardiology, Immunology), stage (Preclinical, Clinical, Post Market Authorization), end-use (Medical Device Companies, Pharmaceutical Companies, Biotechnology Companies), and region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa)
Segment Market Methodology:
The regulatory affairs outsourcing market was segmented into services, company size, category, indication, stage, end-use, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The regulatory affairs outsourcing market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into thirty-eight countries, namely, the U.S.; Canada; the UK; Germany; France; Italy; Spain; Russia; Turkey; Netherlands; Switzerland; Sweden; Europe CIS Countries; Japan; China; India; Australia; South Korea; Indonesia; Malaysia; Singapore; Thailand; Taiwan; CIS Countries; Brazil; Mexico; Argentina; Colombia; Chile; South Africa; Saudi Arabia; UAE; Egypt; Israel.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Regulatory affairs outsourcing market companies & financials:
The regulatory affairs outsourcing market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
Accell Clinical Research, LLC. - Accell Clinical Research, LLC is a privately held international Clinical Research Organization (CRO). It majorly operates in the domestic market, although it also offers services to countries, such as the U.S. Baltic states and Ukraine. The company serves industry participants, such as biotech & pharma producers, manufacturers of nutrition products, and medical device manufacturers. Their U.S. office focuses on biopharmaceutical end-users. The regulatory services have been part of their portfolio since 2014. The company offers services, such as regulatory support, quality assurance, project management, authorization procurement, and clinical supply management. It provides services that comply with local and ICH-GCP guidelines.
-
GenPact Ltd. - Genpact is an international service provider that drives digitally enabled intelligent and digital-led innovation operations for clients, guided by their experience in running thousands of processes for over hundreds of Global Fortune 500 companies. The company operates through various segments that include: healthcare, high tech, life sciences, insurance, commercial banking, retail banking, capital markets, and industrial manufacturing. It serves more than 750 clients across more than 70 countries. The company has 9 operating centers in the U.S., 14 in Europe, and 4 in Japan & Australia. However, Genpact is into pharmaceutical services is gradually trying to enter medical devices market. Its subsidiary Genpact Regulatory Affairs Ltd. is located in the UK. The company enters into various strategic alliances with companies, such as Hitachi and Pharmalink, to increase its product offerings.
-
Criterium, Inc. - Criterium, Inc. is a renowned clinical research organization that provides regulatory consultancy and clinical trial services. In 2012, it ventured into the Indian market to deliver in-country services. They also have presence in countries, such as Mexico, South Africa, Israel, and Russia. The company provides Interactive Voice Response (IVR) services for ensuring higher control and efficiency in clinical studies. The firm’s Oncology Consortia focuses on translational research to develop new cancer therapies.
-
Promedica International - Promedica International is a privately held company providing regulatory and clinical expertise services to biotechnology, pharmaceutical, and medical device companies. The company offers services, such as clinical study management, strategic consulting, medical writing, research compliance & education, and data management. Regulatory services include training programs on good clinical practices, development of clinical Standard Operating Procedures (SOP), and FDA audit support service.
-
WuXi AppTec, Inc. - WuXi AppTec offers an open-access technology platform for medical devices, biopharmaceutical, & pharmaceutical companies with operations in China and the U.S. The company offering includes discovery & development service. Discovery service comprises of discovery chemistry, discovery biology, pharmacology, safety pharmacology, pharmacokinetics, and bioanalysis. Development services include analytical development, commercial manufacturing, and pharmaceutical development. The company also provides preclinical services like toxicology testing, safety pharmacology and bioanalysis. WuXi AppTec. operates as a subsidiary of WuXi PharmaTech (Cayman) Inc. The company also many other subsidiaries through which they cater to specific geographies or categories. For instance, Wuxi Biologics offers regulatory affairs service for Biologics in China.
-
Medpace - Medpace is contract research organization that provides clinical development services for pharmaceutical, biologics, and medical device companies. The company’s clinical development services include management of medical & global regulatory affairs, clinical trial management, medical writing, pharmacovigilance, and core laboratory & quality assurance services. The company’s regulatory affairs services include regulatory strategies for early stage development and regulatory writing for clinical studies. It also provides regulatory approval services and post-market support services, such as literature reviews, clinical study reports, market approvals, vigilance writing, and post-market registries.
-
Charles River Laboratories International, Inc. - Charles River Laboratories International, Inc. (Criver) is the provider of clinical laboratory services and preclinical services for pharmaceutical, and biotechnology industries. Charles operates through three segments: Research Models and Services (RMS), Discovery and Safety Assessment (DSA), and Manufacturing Support. Its DSA segment offerings include bioanalysis, drug metabolism, toxicology, and pharmacokinetics services. It caters its product & services to biotechnology & pharmaceutical companies, medical device companies, hospitals, clinics, and academic institutions.
-
ICON Plc - ICON Plc offers its services to biotechnological, pharmaceutical, government, and public health organizations. It is recognized as the world’s leading provider for contract research organization services. The company operates across 99 locations in 40 countries. In addition, it offers consulting, development, and commercialization services to pharmaceutical industries. ICON plc specializes in the management and analysis of programs that support clinical development.
-
Covance, Inc. - Covance, Inc. is a global contract research organization and offers a vast list of services inclusive of regulatory affairs. Covance drug development includes all the preclinical and clinical Services of discovery and development phase. Services provided by the company are research models, analysis, lead optimization, clinical testing & development, commercialization, and pharmaceutical consulting. The company has presence in around 60 countries. In February 2015, the company was acquired by LabCorp. of American Holdings.
-
PAREXEL International Corporation, Inc. - PAREXEL International Corporation offers services, such as clinical research, medical communications, consulting, advanced technology products & services, and commercialization. These services are used by pharmaceutical, medical device, and biotechnological companies globally. The company operates in three segments, namely PAREXEL Consulting Services, Clinical Research Services (CRS), and PAREXEL Informatics. The services offered by the CRS segment are biostatistics, clinical trial management, clinical logistics, patient disease registries, and observational studies.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Regulatory Affairs Outsourcing Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2022, historic information from 2018 to 2021, and forecast from 2023 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Regulatory Affairs Outsourcing Market Report Assumptions:
-
The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





