- Home
- »
- Pharmaceuticals
- »
-
Skeletal Dysplasia Market Size, Share & Growth Report 2030GVR Report cover
![Skeletal Dysplasia Market Size, Share & Trends Report]()
Skeletal Dysplasia Market (2025 - 2030) Size, Share & Trends Analysis Report By Type (Achondroplasia, X-linked Hypophosphatemia, Hypophosphatasia), By Treatment, By Symptom, By Region, And Segment Forecasts
- Report ID: GVR-3-68038-954-8
- Number of Report Pages: 90
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Skeletal Dysplasia Market Size & Trends
The global skeletal dysplasia market size was valued at USD 3.39 billion in 2024 and is expected to grow at a CAGR of 2.0% from 2025 to 2030. This growth is attributed to increasing awareness and early identification of skeletal dysplasia, which are paramount and supported by educational initiatives from organizations such as the Fetal Medicine Foundation. In addition, technological advancements in treatment, such as gene therapy and targeted medications, contribute significantly. The rising prevalence of congenital disorders and the commercialization of novel drugs are expected to enhance market dynamics.

Skeletal dysplasias, or osteochondrodysplasias, encompass a range of hereditary disorders that disrupt normal cartilage and bone development. This leads to atypical skeletal shapes and sizes, particularly affecting long bones, the spine, and the skull. These conditions arise from genetic mutations impacting specific genes. The skeletal dysplasia market is experiencing growth due to heightened awareness and improved early diagnosis of these disorders. Major pharmaceutical companies are investing in innovative treatments, including gene therapy techniques such as allele silencing and cell-based therapies.
Furthermore, the rising incidence of skeletal dysplasia among children is a significant factor driving market expansion. However, challenges still need to be addressed, particularly in developing regions with limited awareness and diagnostic capabilities. The increasing prevalence of congenital disorders and genetic abnormalities further supports market growth. Public health initiatives and collaborations among research institutions and biotech firms are fostering advancements in treatment options.
However, despite the promising landscape, the market faces hurdles such as a need for comprehensive treatment options and high costs associated with existing therapies. As medical understanding improves and new therapeutic avenues emerge, the skeletal dysplasia market is poised for continued development in the coming years.
Disorder Type Insights
Hypophosphatasia (HPP) dominated the market and accounted for the largest revenue share of 48.5% in 2024 attributed to the increasing awareness and improved diagnostic capabilities, leading to more frequent diagnoses of this rare genetic disorder. In addition, pharmaceutical companies are heavily investing in research and development for innovative treatments, such as enzyme replacement therapies, which are gaining traction. The rising incidence of HPP and a growing demand for early intervention further propel market expansion. Furthermore, affordability and enhanced healthcare access contribute positively to the market's growth trajectory, with a projected CAGR of 5.0% through 2033.
Fibrodysplasia ossificans progressiva (FOP) is expected to grow at a CAGR of 5.6% over the forecast period, owing to the heightened awareness and advocacy efforts from organizations such as the International Fibrodysplasia Ossificans Progressive Association (IFOPA), which promote education about the disease. In addition, the presence of numerous pipeline products aimed at treating FOP is also a significant factor driving market dynamics. Increased research initiatives and collaborations focused on finding effective therapies are expected to enhance treatment options. Furthermore, the rising prevalence of FOP and ongoing clinical trials contribute to a robust market’s growth.
Treatment Insights
Medications led the market and accounted for the largest revenue share of 48.5% in 2024 attributed to the advancements in drug development and increased awareness of skeletal dysplasias. Pharmaceutical companies are focusing on innovative therapies, including enzyme replacement and gene therapies, to address the underlying genetic causes of these disorders. In addition, the rising incidence of congenital conditions has led to a greater demand for effective medications, with treatments like growth hormone therapy showing promise in enhancing bone density and overall health. Furthermore, ongoing research and clinical trials are expanding the range of available therapeutic options, further stimulating market growth.
Surgery is expected to grow at a CAGR of 1.7% over the forecast period driven by driven by patients' growing preference for surgical solutions to address skeletal deformities. As surgical techniques improve and become more specialized, they offer better outcomes for conditions such as achondroplasia and hypophosphatasia. In addition, regulatory approvals for various surgical protocols have bolstered confidence among healthcare providers and patients alike. Furthermore, the increasing awareness of skeletal dysplasia has led to more patients seeking surgical options, contributing to the market's expansion.
Symptom Insights
Skeletal deformities, including parathyroid disorders, growth hormone therapies, and spinal and limb osteotomies, dominated the market and accounted for the largest revenue share of 20.9% in 2024. This growth is driven by increasing awareness and diagnosis rates of skeletal dysplasias, which have led to a higher demand for specialized interventions. In addition, the rising prevalence of congenital disorders necessitates effective treatment options, prompting healthcare providers to adopt innovative surgical approaches. Furthermore, the collaboration between pharmaceutical companies and healthcare institutions further supports the development of targeted therapies, contributing to market expansion.

Dental deformities are expected to grow at a significant CAGR over the forecast period, owing to a growing recognition of the importance of oral health in overall well-being. In addition, increased awareness among healthcare professionals about the relationship between skeletal dysplasia and dental issues has led to more comprehensive treatment plans that include orthodontic care. Innovations in dental technology and materials also facilitate better outcomes for patients with skeletal deformities. Furthermore, as the pediatric population grows, there is a rising demand for early intervention strategies to address dental deformities, ultimately driving market growth.
Regional Insights
North America skeletal dysplasia market is expected to grow substantially over the forecast period. Significant investments in research and development drive this growth. In addition, leading pharmaceutical companies are focused on creating targeted therapies that improve patient outcomes. Increased awareness about rare genetic disorders has led to higher screening and diagnosis rates, fostering a supportive environment for innovative treatments. Furthermore, the prevalence of congenital conditions creates a sustained demand for effective management strategies, further propelling market growth in this region.
U.S. Skeletal Dysplasia Market Trends
The skeletal dysplasia market in the U.S. led the North American market and accounted for the largest revenue share in 2024, owing to increasing awareness of skeletal dysplasias among healthcare providers and the public, leading to more frequent diagnoses and timely interventions. In addition, the presence of leading pharmaceutical companies focused on developing innovative treatments, including enzyme replacement therapies and gene therapies, enhances patient options. Furthermore, government initiatives to address congenital disorders and improve healthcare access further support market growth, as does the rising prevalence of these genetic conditions among the population.
Europe Skeletal Dysplasia Market Trends
Europe skeletal dysplasia market dominated the global market and accounted for the largest revenue share of 39.4% in 2024 attributed to heightened awareness and improved diagnostic capabilities. Organizations focused on educating healthcare professionals and the public about these disorders play a significant role in early identification and intervention. In addition, the region benefits from advanced healthcare systems that support innovative treatment options, including both surgical and pharmaceutical advancements. Furthermore, the increasing prevalence of conditions such as Hypophosphatasia and Fibrodysplasia Ossificans Progressive further drives demand for specialized care, enhancing market dynamics.
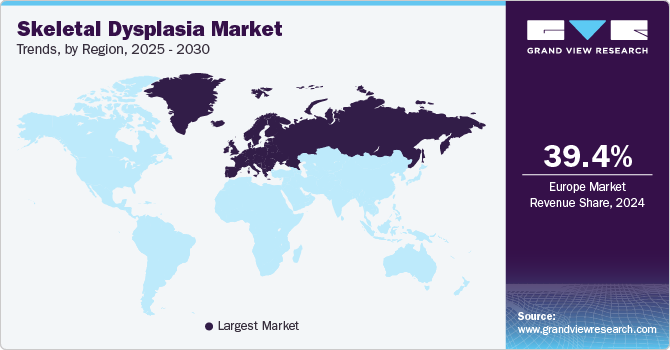
The skeletal dysplasia market in Germany dominated the European market and accounted for the largest revenue share in 2024, owing to its robust healthcare infrastructure and strong emphasis on research. In addition, the country's commitment to genetic testing and early diagnosis enables timely interventions, which are crucial for managing skeletal dysplasias effectively. Furthermore, Germany's focus on developing innovative treatment options, including advanced surgical techniques and targeted therapies, supports market growth. Moreover, the rising incidence of skeletal disorders among children also contributes to the demand for specialized care in this region.
Asia Pacific Skeletal Dysplasia Market Trends
The skeletal dysplasia market in Asia Pacific is expected to grow at a CAGR of 3.3% over the forecast period attributed to rapid advancements in healthcare infrastructure and increasing awareness of rare diseases. Countries such as China and India are enhancing their healthcare expenditures, which improves access to better diagnostic tools and treatment options. In addition, the increasing population correlates with a rise in congenital disorders, driving demand for effective management strategies. Furthermore, collaborative efforts between local healthcare providers and international organizations further enhance this region's market landscape.
Key Skeletal Dysplasia Company Insights
Some of the key companies in the market include Amgen Inc., Merck KGaA, and AstraZeneca plc. Biomarin Pharmaceuticals Inc., and others. These companies adopt various strategies to maintain competitiveness, including strategic collaborations, such as partnerships between pharmaceutical companies and biotechnology firms, to enhance product development capabilities. In addition, new product launches focused on innovative treatments are also vital for capturing market share. Furthermore, mergers and acquisitions allow companies to consolidate resources and expand their portfolios.
-
Merck KGaA actively develops treatments for rare genetic disorders, focusing on skeletal dysplasias such as hypochondroplasia. The company manufactures a range of pharmaceutical products and is engaged in research to advance gene therapies and enzyme replacement therapies. Beyond skeletal dysplasia, the company also operates in oncology, immunology, and neurology segments, contributing to its diverse portfolio.
-
AstraZeneca plc deals in oncology and cardiovascular diseases and explores treatments for rare genetic disorders. The company extensively researches and develops efforts to create targeted therapies for conditions like achondroplasia and other skeletal dysplasias. Furthermore, the company operates in various segments, such as respiratory, inflammation, and autoimmune diseases, enhancing its position in the global healthcare market.
Key Skeletal Dysplasia Companies:
The following are the leading companies in the skeletal dysplasia market. These companies collectively hold the largest market share and dictate industry trends.
- BioMarin
- Amgen Inc.
- Merck KGaA
- Regeneron
- Alexion Pharmaceuticals, Inc.
- Clementia (Ipsen Group).
- AstraZeneca plc.
- Biomarin Pharmaceuticals Inc.
- Cipla Ltd,
- Eli Lilly and Company
- F. Hoffmann-La Roche AG
- Pfizer, Inc.
- Teva Pharmaceutical Industries Ltd.
Skeletal Dysplasia Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 3.49 billion
Revenue forecast in 2030
USD 3.85 billion
Growth Rate
CAGR of 2.0% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD Million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Disorder type, treatment, symptom, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, Russia, India, China, South Korea, Hong Kong, Australia, Singapore, Japan, New Zealand, Thailand, Brazil, Argentina, Kuwait, Saudi Arabia, South Africa, UAE
Key companies profiled
BioMarin; Amgen Inc.; Merck KGaA; Regeneron; Alexion Pharmaceuticals, Inc.; Clementia (Ipsen Group).; AstraZeneca plc.; Biomarin Pharmaceuticals Inc.; Cipla Ltd; Eli Lilly and Company,; F. Hoffmann-La Roche AG; Pfizer, Inc.; Teva Pharmaceutical Industries Ltd.
Customization scope
Free report customization (equivalent to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Skeletal Dysplasia Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and analyzes the latest industry trends in each sub-segment from 2018 to 2030. For this study, Grand View Research has segmented the global skeletal dysplasia market report based on disorder type, treatment, symptom, and region.
-
Disorder Type Outlook (Revenue, USD Million, 2018 - 2030)
-
X-linked Hypophosphatemia (XLH)
-
Hypophosphatasia (HPP)
-
Achondroplasia
-
Fibrodysplasia Ossificans Progressive (FOP)
-
Multiple Osteochondromas (MO)
-
-
Treatment Outlook (Revenue, USD Million, 2018 - 2030)
-
Medication
-
Surgery
-
-
Symptom Outlook (Revenue, USD Million, 2018 - 2030)
-
Skeletal Deformities
-
Dental Deformities
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Russia
-
-
Asia Pacific
-
India
-
China
-
South Korea
-
Hong Kong
-
Australia
-
Singapore
-
Japan
-
New Zealand
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
Kuwait
-
Saudi Arabia
-
South Africa
-
UAE
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










