- Home
- »
- Plastics, Polymers & Resins
- »
-
U.S. Clinical Trial Packaging Market Size, Share Report, 2030GVR Report cover
![U.S. Clinical Trial Packaging Market Size, Share & Trends Report]()
U.S. Clinical Trial Packaging Market (2025 - 2030) Size, Share & Trends Analysis Report By Material (Plastic, Glass, Metal, Paper & Corrugated Fiber), By Product, By End Use, And Segment Forecasts, Key Companies And Competitive Analysis
- Report ID: GVR-4-68040-674-5
- Number of Report Pages: 80
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Bulk Chemicals
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
U.S. Clinical Trial Packaging Market Summary
The U.S. clinical trial packaging market size was estimated at USD 915.9 million in 2024 and is projected to reach USD 1,611.4 million by 2030, growing at a CAGR of 9.9% from 2025 to 2030. The growth is driven by the increasing number of clinical trials, particularly for novel drugs and therapies, including biologics and gene therapies.
Key Market Trends & Insights
- By material, the plastic segment recorded the largest revenue share of over 53.8% in 2024.
- In terms of product segment, the vials & ampoules segment recorded the largest market revenue share in 2024.
Market Size & Forecast
- 2024 Market Size: USD 915.9 Million
- 2030 Projected Market Size: USD 1,611.4 Million
- CAGR (2025-2030): 9.9%
The rising prevalence of chronic diseases necessitates continuous research and development in the pharmaceutical and biotechnology sectors, leading to a higher demand for specialized packaging solutions to ensure drug stability, safety, and integrity throughout the trial phases. Increasing regulatory scrutiny and the complexity of trial protocols necessitate secure and adaptive packaging formats. The growth of the U.S. clinical trial packaging industry is primarily driven by the rising number of clinical trials, spurred by growing investments in pharmaceutical R&D and the surge in novel drug development.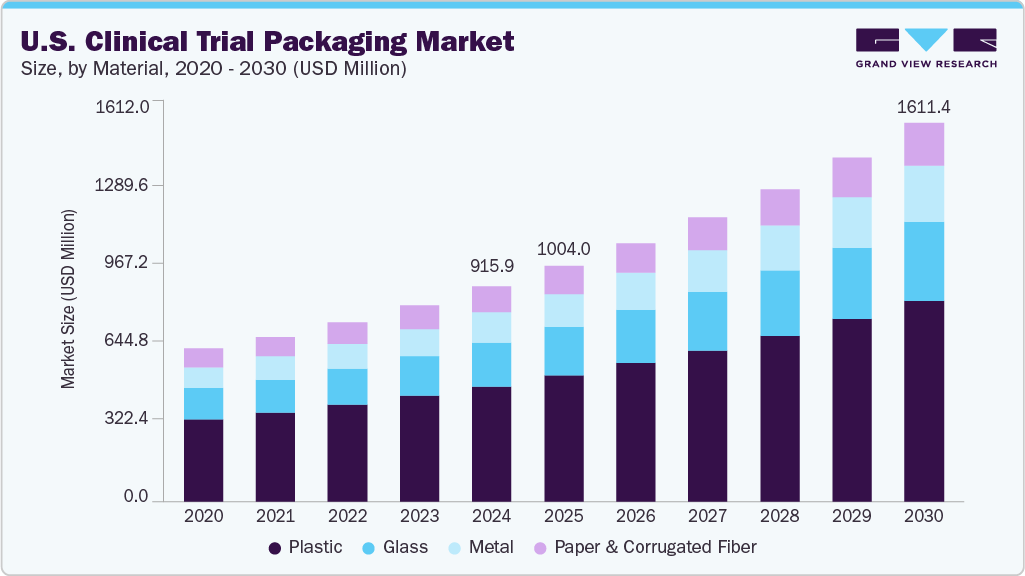
Innovation is a cornerstone of the U.S. clinical trial packaging industry, continuously driven by the need for enhanced drug stability, patient compliance, and supply chain efficiency. Companies invest in smart packaging technologies, such as RFID and temperature monitoring devices, to provide real-time data on product conditions and ensure integrity throughout cold chain logistics.
Material Insights
The plastic material segment held the largest revenue share of 53.8% in 2024, largely due to its versatility, cost-effectiveness, and ability to be customized for various drug forms, including solid doses, liquids, and semi-solids. It is commonly used for bottles, pouches, and vials. The increasing need for sterile, single-use packaging solutions in trials and rising clinical research activities further enhances plastic's dominance.
The metal segment is expected to grow at the fastest CAGR of 10.8% over the forecast period. This growth is attributed to the superior barrier properties of metal, offering robust protection against light, moisture, and oxygen, which is critical for highly sensitive or high-value drug products.Metal packaging provides exceptional durability and tamper-evident features, crucial for maintaining product integrity and security throughout the complex clinical trial supply chain.
Product Insights
The vials & ampoules segment held the largest revenue share in 2024 and is expected to grow at the fastest CAGR over the forecast period. The growth is due to their versatility in holding lyophilized, liquid and powder-based drugs. Vials offer reusability and resealing features, while ampoules offer a tamper-proof single-use solution. Growth in parenteral drug development and temperature-sensitive biologics also fuels the need for durable, inert packaging such as vials and ampoules.
The bags and pouches segment is expected to grow at a significant CAGR over the forecast period. requirements make them an increasingly preferred choice among clinical research organizations and sponsors seeking compliance and logistical efficiency.
End Use Insights
The research laboratories segment dominated the market in 2024. Research laboratories are key stakeholders in early-stage drug development and are responsible for preclinical and clinical testing. These laboratories require specialized clinical trial packaging solutions to ensure sample integrity, compliance with regulatory standards, and efficient identification during complex trials.

The clinical research organizations segment is expected to experience the fastest CAGR over the forecast period.This rapid growth is fueled by the increasing trend of pharmaceutical and biotechnology companies outsourcing their clinical trial management, including specialized packaging, labeling, and distribution services, to CROs. They offer expertise and infrastructure to manage complex supply chains, cold chain logistics, and regulatory compliance for clinical trial materials.
Key U.S. Clinical Trial Packaging Company Insights
Some of the key players operating in the market include Catalent, Inc., PCI Pharma Services, Xerimis, and others.
- Catalent provides advanced delivery technologies and development and manufacturing solutions for drugs, biologics, gene therapies, and consumer health products. It offers comprehensive clinical supply services for clinical trials, including secondary packaging and clinical labeling, often performed in temperature-controlled environments.
Key U.S. Clinical Trial Packaging Companies:
- Catalent, Inc.
- PCI Pharma Services
- Parexel International (MA) Corporation.
- Piramal Pharma Limited
- Rubicon Research Limited
- Schreiner Group
- Xerimis
Recent Developments
- In September 2024, PCI Pharma Services announced a USD 365 million investment in European Union and the U.S. facilities for clinical and commercial scale supply of advanced drug device combination and drug delivery products.
U.S. Clinical Trial Packaging Market Report Scope
Report Attribute
Details
Revenue forecast in 2030
USD 1,611.4 million
Growth Rate
CAGR of 9.9% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Material, product, end use
Key companies profiled
Catalent, Inc.; PCI Pharma Services; Parexel International (MA) Corporation: Piramal Pharma Limited; Rubicon Research Limited; Schreiner Group; Sharp Services, LLC; Xerimis
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
U.S. Clinical Trial Packaging Market Report Segmentation
This report forecasts revenue growth at U.S. level and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the U.S. clinical trial packaging industry report based on material, product, and end use:
-
By Material Outlook (Revenue, USD Million, 2018 - 2030)
-
Plastic
-
Glass
-
Metal
-
Paper & Corrugated Fiber
-
-
By Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Syringes
-
Vials & Ampoules
-
Bottles
-
Bags & Pouches
-
Others
-
-
By End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Research Laboratories
-
Clinical Research Organizations
-
Drug Manufacturing Facilities
-
Frequently Asked Questions About This Report
b. The U.S. clinical trial packaging market was estimated at around USD 915.9 million in the year 2024 and is expected to reach around USD 1,004.0 million in 2025.
b. The U.S. clinical trial packaging market is expected to grow at a compound annual growth rate of 9.9% from 2025 to 2030 to reach around USD 1611.4 million by 2030.
b. Research laboratories emerged as the dominating end-use segment in the clinical trial packaging market due to their high involvement in early-phase trials and frequent handling of diverse sample types. Their need for specialized, secure, and compliant packaging drives demand in this segment.
b. The key players in the U.S. clinical trial packaging market include Fisher Clinical Services, PCI Pharma Services, PAREXEL, Schreiner, MediPharm, Sharp Packaging, The Coghlan Group, Rubicon, Xerimis, Catalent, and Piramal Pharma Solutions
b. The U.S. clinical trial packaging market is driven by the increasing number of clinical trials globally and the rising demand for specialized, tamper-evident, and regulatory-compliant packaging. Growth in biologics and personalized medicine further fuels the need for advanced packaging solutions.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










