- Home
- »
- Pharmaceuticals
- »
-
Vaccine Market Size, Share, Growth & Trends Report, 2030GVR Report cover
![Vaccine Market Size, Share & Trends Report]()
Vaccine Market Size, Share & Trends Analysis Report By Type (Subunit, mRNA), By Route Of Administration (Oral), By Disease Indication (HPV, MMR), By Age Group (Adult), By Distribution Channel, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-990-6
- Number of Report Pages: 180
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Vaccine Market Size & Trends
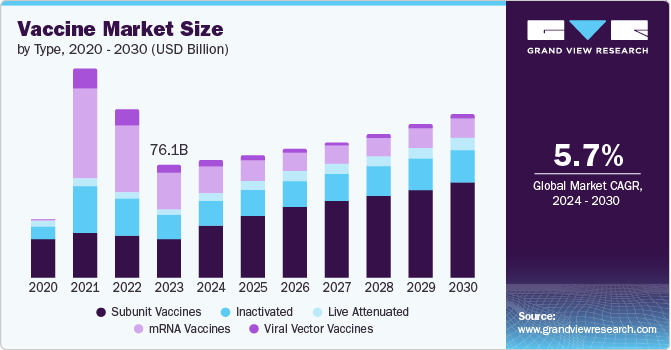
The global vaccine market size was estimated at USD 76.08 billion in 2023 and is expected to grow at a CAGR of 5.74% from 2024 to 2030. The vaccine market has been expanding globally, with an increase in vaccines targeting diseases that predominantly affect lower-income countries. In the U.S., the COVID-19 vaccine market transitioned to a commercial phase, following the depletion of the federal government's purchased stock. This shift will likely result in higher prices, as evidenced by Moderna's announcement in March 2023 that its COVID-19 vaccine price would rise to approximately USD 110 to USD 130 per dose. The privatization of the market will also intensify competition among manufacturers post pandemic.
In March 2023, the Serum Institute of India announced its plans to diversify beyond COVID-19 vaccines by developing new vaccines for malaria and dengue. Company officials, stated that the company has repurposed its COVID-19 vaccine manufacturing facilities to produce these new vaccines, potentially increasing its total production capacity to 4 billion doses annually. This strategic move allows Serum Institute to maintain high production levels and ensure rapid response capability in case of future pandemics.
By March 2024, Dr. Reddy’s Laboratories (DRL) will begin promoting and distributing Sanofi’s vaccine brands in India. This partnership includes well-known pediatric and adult vaccines such as Hexaxim, Pentaxim, Tetraxim, Menactra, FluQuadri, Adacel, and Avaxim 80U. These brands achieved combined sales of about USD 51 million as of February 2024. This collaboration strengthens DRL’s vaccine portfolio, positioning it as the second-largest vaccine player in India, while Sanofi continues to own, manufacture, and import these vaccines into the country.
The COVID-19 pandemic highlights the limitations of relying solely on public investment and procurement strategies for optimal public health outcomes. Although around 15 billion vaccine doses were globally distributed by October 2022, only 12% were provided through COVAX, the global alliance for equitable access. This highlights the need for more comprehensive approaches to achieve equitable distribution and prepare for future pandemics.
Regulatory agencies and regional networks have been crucial in enhancing regulatory capacity and promoting coordinated efforts across countries, leading to better accessibility of new vaccines. The World Health Organization (WHO) has been instrumental in this regard, providing regulatory assistance through its prequalification program and helping countries develop efficient, stable, and integrated regulatory systems. As a result, the national regulatory authorities in 35 vaccine-producing countries have achieved a maturity level sufficient to oversee development, manufacturing, and release.
Industry Dynamics
Technology trends are increasingly focused on improving vaccine development processes and accessibility. There are significant innovations in formulations, storage, and distribution methods aimed at enhancing effectiveness and streamlining delivery to diverse populations. In addition, user-friendly digital tools are being integrated to monitor vaccination coverage and address supply chain challenges. This technological advancement is promoting a more efficient and widespread impact in the fight against diseases such as pneumonia and other diseases. These trends are expected to continue shaping the market, ensuring better health outcomes globally.
The space is experiencing steady growth and moderate merger and acquisition (M&A) activity among leading players. In May 2024, Novavax and Sanofi announced a co-exclusive licensing agreement to co-commercialize a COVID-19 vaccine and to develop new COVID-19-influenza combination vaccines. This partnership exemplifies the strategic collaborations taking place in the industry as companies seek to expand their product offerings and improve market reach. Such alliances are expected to drive innovation and provide comprehensive solutions for infectious diseases. This M&A activity reflects the broader trend of consolidation in the vaccine market, aimed at enhancing competitive advantage and addressing public health needs more effectively.
The regulatory trend in the vaccine market indicates a growing emphasis on ensuring vaccine safety, efficacy, and accessibility. Governments worldwide are actively working on streamlining approval processes, monitoring vaccine distribution, and encouraging manufacturers to meet quality standards. These efforts aim to support public confidence, facilitate timely vaccine availability, and enhance the overall effectiveness of vaccination programs.
The threat of substitutes in the vaccine market is relatively low. Vaccines are a critical component of public health, and there are few alternatives that offer the same level of effectiveness in preventing diseases. While treatments and therapies exist for some illnesses, they generally do not provide the same preventative benefits as vaccines. Moreover, the high efficacy, long-lasting protection, and ability to prevent outbreaks make vaccines the preferred choice for both healthcare providers and patients. In addition, government support and public health policies often mandate or strongly recommend vaccinations, further reducing the likelihood of substitutes gaining significant market share.
Companies are increasingly adopting strategies to expand their product reach and availability across diverse regions. Leading players such as SINOVAC, CSL Seqirus (CSL Limited), and Sanofi are at the forefront of these efforts. For instance, in April 2023, SINOVAC opened a new vaccine production facility in Beijing to address the growing global demand for high-quality influenza vaccines. By establishing new facilities and enhancing production capabilities, these companies aim to increase their market presence and ensure their vaccines are accessible to a wider population. This approach not only meets the rising demand but also strengthens their position in the global vaccine market.
Type Insights
The mRNA segment dominated the vaccine market with a share of 32.32% in 2023. In the space Pfizer/BioNTech and Moderna have gained prominence with their mRNA COVID-19 vaccines. These vaccines offer significant advantages over traditional vaccines, such as the ability to quickly adjust antigen design and integrate sequences from multiple variants to address new mutations in the virus genome. This adaptability has been a key factor in their market dominance. The success of mRNA-based COVID-19 vaccines has also spurred the development of mRNA platforms for preventing other infectious diseases such as flu and RSV. For instance, in February 2023, Moderna announced interim results from its phase 3 trial for mRNA-1010, a seasonal flu shot. The vaccine showed superior results against influenza A strains but was less effective against certain influenza B strains. This ongoing research highlights the potential of mRNA technology to revolutionize the vaccine market further.
The subunit vaccines segment is expected to experience rapid growth during the forecast period. This growth is driven by several factors, including the increasing prevalence of infectious diseases, rising demand for safe and effective vaccines, and a growing emphasis on preventive healthcare. Moreover, there is a rising need for more efficient vaccines targeting diseases such as cancer, autoimmune disorders, and allergies. For instance, in November 2022, Curevo Vaccine (Curevo), a biotechnology company focusing on developing vaccines to combat infectious diseases, completed a Series A1 funding round worth USD 26 million. The funding will support the development of CRV-101, an adjuvanted subunit vaccine aimed at preventing shingles in older adults. This investment reflects the growing interest and investment in subunit vaccines to address various health challenges, contributing to the segment's projected growth in the vaccine market.
Route Of Administration
Parenteral administration is highly preferred for administering vaccines and hence the segment dominated the vaccine market with a share of 97.01% in 2023. Parenteral administration, which involves injections into the body, is preferred for its faster absorption, higher efficacy, and reduced risk of contamination and degradation. Currently, majority of vaccines on the market are administered intramuscularly or subcutaneously, which contributes to the dominance of this segment.
However, the oral administration segment is expected to grow steadily, over the forecasted period. This growth is attributed to the advantages of orally administered vaccines, such as the stimulation of both mucosal and systemic immunities, and the elimination of adverse events associated with injections. Vaccines such as polio and rotavirus, particularly used in pediatric vaccinations, are commonly administered orally, further driving the growth of this segment.
Disease Indication Insights
The viral diseases segment is further segmented into Hepatitis, Influenza, HPV, MMR, Rotavirus, Herpes Zoster, COVID-19 and others. This segment dominated the vaccine market with a share of 63.79% in 2023, majorly attributed to COVID-19 vaccines. The market is driven by factors such as increasing the incidence of viral diseases, growing awareness about the benefits of vaccination, and government initiatives to promote immunization programs. The market is highly competitive and is dominated by major players such as Pfizer, GSK, AstraZeneca and Serum Institute amongst others.
The market for bacterial diseases is expected to grow at a fastest CAGR during the forecast period. The growing demand for vaccination that can prevent bacterial infections, particularly in regions with high incidences of bacterial diseases is one of the major factors driving segment growth. This market includes the development and distribution of vaccination for a range of bacterial infections, such as pneumonia, meningitis, DPT, and others. In February 2023, BactiVac, the Bacterial Vaccines Network, received USD 1.25 million in funding from Wellcome to accelerate the development of bacterial vaccines and counteract the threat of antimicrobial resistance (AMR). The funding will be utilized to amplify the impact of the BactiVac Network during the next four years.
Age Group Insights
Adult accounted for a share of 57.25% in the vaccine market in 2023. Adult vaccination, including those for COVID-19, comprised 75% in terms of volume globally, while pediatric vaccines accounted for about 20%. In comparison to pre-Covid, adult vaccine volumes had a nine-fold increase primarily due to COVID-19 vaccination. In addition, non-COVID-19 adult vaccine volumes increased by 15% because of widespread use of seasonal influenza vaccination in high-income countries (HICs).
Pediatric segment is estimated to be the fastest growing segment over the forecast period. During the COVID-19 pandemic, overall pediatric vaccines volumes decreased by 14% compared to the pre-pandemic, which was driven by a decreased use of oral polio vaccine (OPV) and measles-rubella vaccine (MR) in supplemental immunization activities (SIAs). The approval of COVID vaccines of pediatric use will further boost segment growth. For instance, in June 2022, Pfizer/BioNTech COVID vaccine received emergency use approval (EUA) for use in children aged 6 months to 4 years of age.
Distribution Channel Insights
Government suppliers dominated the vaccine market in 2023 with a share of 59.90%. In contrast to other pharmaceutical products, vaccines are primarily financed by public funds through government and pooled procurement arrangements, with private sector procurement playing a minor role. Concentrated demand can assist in planning the necessary supply investments. However, the predictability of demand remains a significant factor affecting access to vaccinations. For instance, the U.S. government has recently announced that it planned to purchase more than 1.5 million Novavax Inc's COVID-19 vaccine doses in February 2023. As per the recent agreement, funds have been allocated for the development of an updated vaccine, expected to be ready by fall of the same year.
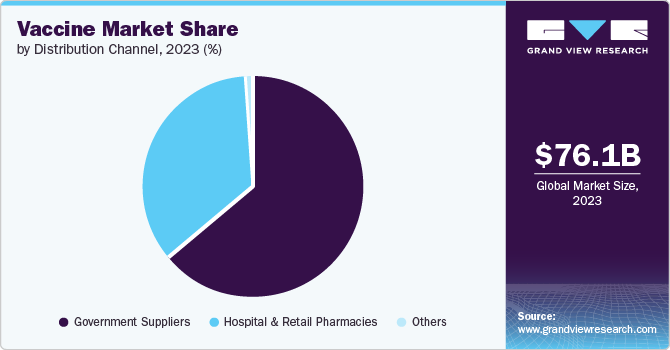
Hospital & retail pharmacies supply channel is estimated to be the fastest growing distribution channel in the vaccine market. Moreover, pharmacies have become an increasingly important source of vaccine supply in many developed countries. They provide convenient access to public vaccination and help increase vaccination rates. Furthermore, the opening of the private market for COVID vaccines in 2023 in the U.S. will also contribute to the market growth.
Regional Insights
North America vaccines market is estimated to grow at a fastest CAGR of 8.42% over the forecast period. Government initiatives promoting immunization and heightened awareness post-pandemic drive adoption. For instance, the Hepatitis B Foundation strongly supports the updated adult hepatitis B vaccination recommendations from the U.S. Centers for Disease Control and Prevention (CDC). The Foundation is actively coordinating with a panel of experts to facilitate the effective implementation of these guidelines, aiming to enhance vaccination coverage against this dangerous virus among millions of U.S. adults. In the U.S., chronic hepatitis B affects around 2.4 million individuals, with thousands dying due to the disease annually. Left untreated, chronic hepatitis B carries a significant 25% to 40% lifetime risk of developing the often fatal condition of liver cancer.
U.S. Vaccines Market Trends
The vaccines market in the U.S. accounted for the major revenue share of the global vaccine market in 2023. Rapid technological advancements, recent U.S. FDA approvals for influenza vaccine, and intense competition between companies are expected to boost the market over the forecast period. For instance, in June 2022, CSL Seqirus announced the completion of a USD 156 million expansion at its production facility in the U.S. This expansion is expected to support the formulation of its cell-based influenza vaccines in prefilled syringes. In addition, in October 2021, Seqirus announced U.S. FDA approval of its quadrivalent influenza vaccine FLUCELVAX QUADRIVALENT. This newly approved vaccine has an expanded age indication for children as young as 6 months old. Moreover, this product is the first and only cell-based influenza vaccine approved in the U.S.
Europe Vaccines Market Trends
Europe vaccines market held the second-largest share in 2023, following Asia Pacific over the forecast period. The market growth can be attributed to an increase in research funding and the presence of local key market players in this region. The number of biopharmaceutical companies is growing in Europe owing to increasing investments. For instance, in February 2022, the UK pledged USD 192 million to the Coalition for Epidemic Preparedness Innovations to boost vaccine development.
The vaccine market in the UK is one of the major markets in the region. High vaccine uptake against the flu, better immunization programs, favorable government initiatives, and increased R&D activities in the country are driving the market growth. Following this development, in January 2024, Pfizer gained a positive opinion from the CHMP for its 20-Valent Pneumococcal Conjugate Vaccine (20vPnC). This vaccine offers broad coverage against pneumococcal disease, encompassing all 20 serotypes contained within the vaccine. These serotypes represent a significant portion of circulating pneumococcal disease globally and in the EU.
The France vaccine market is growing majorly due to awareness programs conducted by various government and non-government organizations. France launched a flu vaccine campaign to spread awareness regarding flu and influenza in the country. Such awareness programs help people identify symptoms, thereby creating more demand for influenza vaccines. France has a blend of several regional and multinational players offering influenza vaccines. Thus, several strategic initiatives undertaken by leading participants are likely to fuel market expansion.
The vaccine market in Germany dominated the European region owing to the presence of research institutions and leading vaccine manufacturers. In addition, a surge in R&D activities and various strategic initiatives undertaken by leading participants are factors propelling market expansion.
Moreover, in November 2022, BioNTech SE & Pfizer, Inc. initiated a phase I trial for a single-dose mRNA-based combination vaccine candidate for COVID-19 and influenza. In addition, in recent years, Bavarian Nordic A/S established a collaboration with Dynavax Technologies Corporation, a biopharmaceutical firm dedicated to creating and promoting innovative vaccines. This partnership aims to facilitate the marketing and distribution of HEPLISAV B [Hepatitis B Vaccine (Recombinant), Adjuvanted] in Germany.
Asia Pacific Vaccines Market Trends
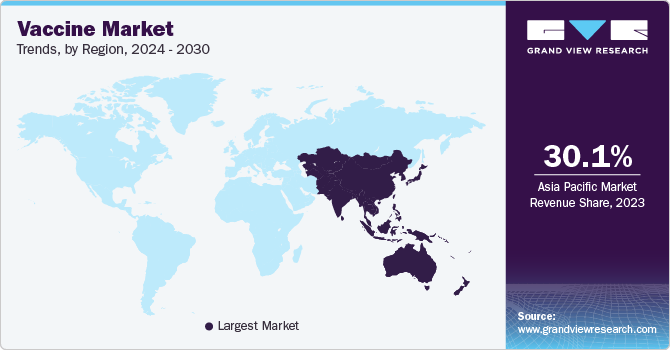
The vaccine market in Asia Pacific held the largest share of 30.86% in 2023. The vaccine market in Asia Pacific is expected to grow at the fastest rate over the forecast period due to various factors, including improving healthcare reforms. Some of the other factors contributing to market growth are increasing geriatric population, improving healthcare infrastructure, and the entry of new players.
Some of the major players operating in Asia Pacific market are GSK plc, AstraZeneca, Sanofi, and Serum Institute of India (SII). In addition, owing to the region’s large population, the rate of influenza transmission is also higher. For instance, according to the Singapore Ministry of Health, the country has a higher burden of respiratory infections such as influenza than other diseases.
The vaccines market in India is driven by increasing prevalence of influenza in the country, coupled with several awareness campaigns arranged by local governments. India has a flourishing pharmaceutical sector owing to the emergence of generic manufacturers and the presence of leading pharmaceutical players. In addition, rising government efforts to bring next-generation influenza vaccines into the country is another factor propelling the country’s market.
The China vaccines market is experiencing steady growth driven by the local presence of a large number of vaccine manufacturers, favorable government initiatives, and high disease burden due to its large population. In addition, a supportive regulatory framework and high demand for innovative vaccines are expected to offer lucrative growth opportunities.
The vaccines market in Japan is one of the most technologically advanced market in Asia Pacific. Its vaccine market is expected to grow rapidly over the forecast period owing to high government spending for reducing the flu burden. Multiple initiatives, such as government grants to various research institutes and companies, can help develop practical solutions for the influenza burden in the country. In September 2023, Pfizer Japan filed for the approval of its 20-valent pneumococcal conjugate vaccine, Apexxnar, targeting older adults and individuals at high risk of contracting pneumococcal infections. This vaccine aims to provide broader protection by covering seven additional serotypes beyond Prevenar 13, Pfizer's existing pneumococcal vaccine.
Latin America Vaccines Market Trends
The Latin America vaccine market exhibited significant growth in the past few years owing to the high disease burden and increased demand for infectious disease vaccines. Mexico, Brazil, and Argentina are the major markets for influenza vaccines in Latin America. Favorable government initiatives and research collaborations are anticipated to contribute to the market growth. In addition, the demand for influenza vaccines increased owing to the current disease burden and high demand from healthcare providers.
The vaccine market in Brazil is significantly driven by supportive government initiatives to promote vaccine supply and uptake. For instance, in July 2022, Emergex Vaccines Holding Limited announced a partnership with the Molecular Biology Institute of Paraná (IBMP) in Brazil. The IBMP, a national organization for research of vaccines, is linked to the Health Ministry of Brazil. This agreement allowed IBMP to gain official rights for the commercialization of novel vaccines in Brazil.
MEA Vaccines Market Trends
The MEA vaccines market is expected to grow owing to economic development witnessed in emerging markets, such as South Africa, highly unmet healthcare needs, and high prevalence of diseases. Moreover, the increased prevalence of flu and the risk of seasonal influenza are propelling the market growth in the region.
Influenza vaccination can help prevent influenza-related illness, even in people who are at high risk of developing severe complications from the virus. This propelled many organizations, such as the WHO, the Agency of Preventive Medicine, and Ministry of Health in Africa, to collaborate for monitoring & evaluating the occurrence of influenza among the population and initiate vaccination programs in the region.
The vaccines market in Saudi Arabia, despite having a low local output of branded drugs relative to generic products, Saudi Arabia has the largest pharmaceutical market in the Middle East and African region. Moreover, the country is experiencing an increased prevalence of influenza. According to the Saudi Ministry of Health, seasonal influenza causes a variety of complications, the most serious of which are bronchitis, pneumonia, blood poisoning, ear infection, and death. As a result, in October 2022, the Ministry of Health launched an awareness campaign to encourage seasonal influenza vaccination, focusing on groups most vulnerable to it, such as the elderly, people with chronic diseases or immunodeficiency, healthcare workers, and pregnant women. The ministry emphasized that vaccination is safe, has few adverse effects, and has been proven to be effective around the world. Such initiatives are expected to increase vaccination rates in the country and propel the market growth.
Key Vaccine Company Insights
The vaccine market is highly competitive, with a few large players. The supply landscape underwent a significant change amid the pandemic. Many manufacturers entered the market to capture the global share, however, the top 10 manufacturers accounted for almost 90% of the global value, reflecting greater market concentration than all other vaccines.Companies are engaged in development of vaccines using new technology for diseases such as RSV, flu, and influenza.
Key Vaccine Companies:
The following are the leading companies in the vaccine market. These companies collectively hold the largest market share and dictate industry trends.
- Serum Institute of India Pvt. Ltd.
- Seqirus
- Sanofi
- GSK Plc.
- Merck & Co., Inc.
- Pfizer Inc.
- Moderna Inc.
- Sinovac
- BioNTech SE
- AstraZeneca
Recent Developments
- In May 2024, Novavax and Sanofi disclosed a co-exclusive licensing agreement to co-commercialize a COVID-19 vaccine and collaborate on developing novel combination vaccines for COVID-19 and influenza. This strategic partnership allows both companies to leverage their respective expertise and resources to bring innovative vaccine solutions to the market. By joining forces, Novavax and Sanofi aim to enhance their competitive position in the rapidly evolving landscape of vaccine development and distribution. The agreement signifies a proactive approach to addressing public health challenges and meeting the increasing demand for effective vaccines against infectious diseases.
- In April 2023, Pfizer announced data from phase 3 trials of its RSV shots. The trial conducted in adult patients demonstrated that the shot was 67% more effective in preventing infections with two related symptoms and 86% effective in case of severe disease. The successful approval of this vaccine will make it the first product in the RSV market.
- In March 2023, CSL Limited inaugurated a new vaccine R&D facility in Waltham, Massachusetts, to develop advanced vaccines by using disruptive technologies such as next-generation mRNA.
Vaccine Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 79.09 billion
Revenue forecast in 2030
USD 110.54 billion
Growth rate
CAGR of 5.74% from 2024 to 2030
Actual years
2018 - 2023
Forecast period
2023 - 2030
Report update
July 2024
Quantitative units
Revenue in USD billion and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, route of administration, disease indication, age group, distribution channel, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Thailand; Brazil; Mexico, Argentina; South Africa; Saudi Arabia, UAE; Kuwait
Key companies profiled
Serum Institute of India Pvt. Ltd.; Seqirus; Sanofi; GSK Plc.; Merck & Co. Inc.; Pfizer Inc.; Moderna Inc.; Sinovac; BioNTech SE; AstraZeneca
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Global Vaccine Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global vaccine market on the basis of type, route of administration, disease indication, age group, distribution channel, and region:
-
Type Scope Outlook (Revenue, USD Billion, 2018 - 2030)
-
Subunit Vaccines
-
Recombinant vaccines
-
Conjugate Vaccines
-
Toxoid vaccines
-
-
Inactivated
-
Live Attenuated
-
mRNA vaccines
-
Viral vector vaccines
-
-
Route of Administration Scope Outlook (Revenue, USD Billion, 2018 - 2030)
-
Oral
-
Parenteral
-
Nasal
-
-
Disease Indication Scope Outlook (Revenue, USD Billion, 2018 - 2030)
-
Viral Diseases
-
Hepatitis
-
Influenza
-
HPV
-
MMR
-
Rotavirus
-
Herpes Zoster
-
Covid-19
-
Others
-
-
Bacterial Vaccines
-
Meningococcal Diseases
-
Pneumococcal diseases
-
DPT
-
Others
-
-
Cancer Vaccines
-
Allergy Vaccines
-
-
Age Scope Outlook (Revenue, USD Billion, 2018 - 2030)
-
Pediatric
-
Adult
-
-
Distribution Channel Scope Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospital & Retail Pharmacies
-
Government Suppliers
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global vaccine market size was estimated at USD 76.08 billion in 2023 and is expected to reach USD 79.09 billion in 2024.
b. The global vaccine market is expected to grow at a compound annual growth rate of 5.74% from 2024 to 2030 to reach USD 110.54 billion by 2030.
b. The viral diseases segment dominated the vaccine market with a share of 81.42% in 2023. This is attributable to the increasing incidence of viral diseases, growing awareness about the benefits of vaccination, and government initiatives to promote immunization programs.
b. Some key players operating in the vaccine market include Merck &Co., Inc., Emergent BioSolutions, Inc., Johnson and Johnson Services, Inc., Sanofi, Pfizer, Inc., Novartis AG, CSL Ltd., and GlaxoSmithKline plc.
b. Key factors that are driving the market growth include rising demand for better healthcare infrastructure and high awareness levels of the benefits of immunization.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





