- Home
- »
- Market Trend Reports
- »
-
Omics-Based Clinical Trials: Current Dynamics And Pipeline Outlook
Report Overview
Omics technologies encompassing genomics, proteomics, metabolomics, transcriptomics, and epigenomics have transformed clinical trials by enabling a deeper understanding of biological variability among patients. These approaches allow researchers to identify molecular biomarkers that predict drug response, disease progression, and patient stratification more accurately than traditional methods. The rise of personalized medicine depends heavily on omics-driven trials, which tailor interventions to genetic and molecular profiles. For example, genomics-guided clinical trials in oncology have improved patient selection for targeted therapies, reducing adverse events and improving efficacy.
Omics-Based Clinical Trials Current Dynamics and Pipeline Outlook Report Coverage
Omics-Based Clinical Trials: Current Dynamics and Pipeline Outlook Report Coverage
Market Outlook
Prevalence Trends Analysis
R&D Investment Analysis
Industry Ecosystem Analysis
Market Dynamics
Regulatory Framework
List of Top 100 Active Trials by Phase, Sponsor, and Indication
Emerging Clinical Trial Model Analysis
Global Omics-Based Clinical Trials, by Phase & Study Design
Global Omics-Based Clinical Trials, by key Indications, By Region
Furthermore,growing government initiatives have accelerated the integration of omics into clinical trials by providing funding, infrastructure, and regulatory frameworks. The U.S. National Institutes of Health (NIH) launched the All of Us Research Program, which aims to gather genomic and health data from over one million diverse participants to drive precision medicine. Similarly, the European Union’s Horizon Europe program funds projects focused on multi-omics data integration to enhance disease understanding and treatment outcomes. Regulatory agencies, including the FDA and EMA, have published guidance documents encouraging the use of biomarkers in trial design and drug approval pathways, facilitating smoother adoption. The increased availability of large-scale biobanks and public genomic datasets has further reduced barriers to omics research in trials. Companies such as Illumina and Thermo Fisher Scientific provide next-generation sequencing platforms that are increasingly deployed in clinical settings to enable these studies, reflecting the technological advancements supporting this trend.
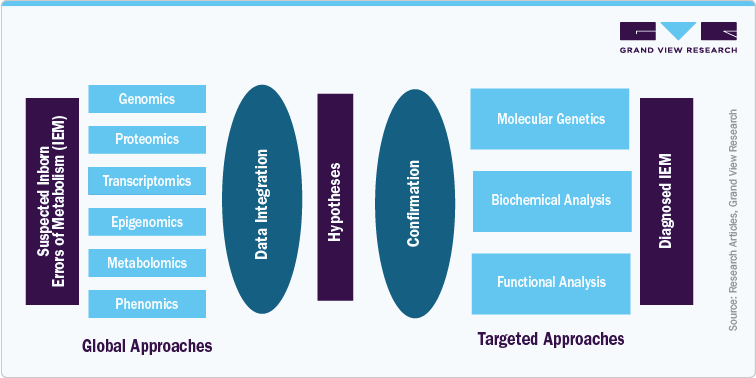
Omics-based clinical trials are rapidly evolving as a cornerstone of precision medicine, driven by advances in high-throughput sequencing, biomarker discovery, and multi-dimensional data integration. These trials no longer rely solely on traditional clinical endpoints but incorporate genomic, transcriptomic, proteomic, and metabolomic data to design more targeted, efficient, and adaptive studies. This shift is particularly important in areas such as rare diseases, oncology, and metabolic disorders, where patient heterogeneity and molecular complexity demand deeper biological insights. For instance, the diagnostic framework for Inborn Errors of Metabolism (IEM) depicted in recent multi-omics workflows illustrates how global approaches like genomics and metabolomics are used to generate hypotheses, which are then confirmed through targeted molecular and biochemical analyses.
R&D Investment and Funding Analysis
Investment in research and development for omics-based clinical trials has seen a consistent upward trajectory driven by the promising potential of precision medicine. Governments, academic institutions, and private sector players recognize the need to fund studies that harness high-throughput omics technologies for improved patient outcomes. Public funding agencies such as the U.S. National Institutes of Health (NIH) allocated approximately USD 6 billion annually toward genomics and related biomedical research, reflecting strong governmental commitment to advancing omics science.
This financial support enables large-scale cohort studies and infrastructure development, such as biobanks and sequencing facilities, that are crucial for robust clinical trials. Additionally, venture capital investments in biotechnology companies focusing on omics-driven diagnostics and therapeutics have grown significantly, signalling strong industry confidence. For instance, in recent years, companies like 23andMe and Guardant Health have secured hundreds of millions in funding rounds to expand their genomic testing and liquid biopsy capabilities, respectively.

The ecosystem of omics-based clinical trials encompasses a diverse array of participants working in concert to advance precision medicine across multiple therapeutic areas. Central to this ecosystem are pharmaceutical and biotech companies that leverage omics insights to design targeted therapies tailored to patients’ molecular profiles. Complementing these efforts, diagnostic firms deliver essential genomic and proteomic testing services that enable precise patient selection and real-time monitoring during trials.
Academic institutions contribute critical research expertise and serve as key sites for trial execution, fostering innovation in biomarker discovery and validation. Technology providers supply cutting-edge platforms for high-throughput sequencing and multi-omics data generation, while bioinformatics companies develop sophisticated computational tools that transform complex datasets into actionable clinical insights.
Contract research organizations (CROs) adapt trial operations to accommodate the specialized demands of omics-driven studies, ensuring regulatory compliance and efficient data management. Regulatory agencies play a pivotal role by establishing frameworks that support the integration of omics biomarkers into clinical development pathways. Government funding and international collaborations further enhance the ecosystem by building infrastructure, enabling large-scale data sharing, and promoting standardization. This collaborative network of stakeholders collectively accelerates the translation of omics innovations into effective, personalized therapies, shaping the future landscape of clinical trials.

Emerging clinical trial models in omics-based research are reshaping how studies are designed and conducted to better capture the complexity of human biology and improve therapeutic outcomes. Adaptive trial designs are gaining traction by allowing modifications to protocols based on interim omics data, such as genomic alterations or biomarker responses, which enable dynamic patient stratification and more efficient resource utilization. Basket trials, which group patients by shared molecular markers rather than tumor location or disease subtype, exemplify how omics data drives more precise and inclusive study populations. Similarly, umbrella trials test multiple targeted therapies within a single disease based on diverse molecular profiles, accelerating identification of effective treatments.
Decentralized or virtual trial models increasingly leverage digital health technologies and remote patient monitoring, facilitating broader and more diverse patient recruitment while integrating real-world omics data. Multi-omics integration in trial designs allows simultaneous analysis of genomics, transcriptomics, proteomics, and metabolomics, providing a holistic view of disease mechanisms and treatment response. These novel approaches improve the statistical power and flexibility of clinical studies and reduce timelines and costs. By harnessing high-resolution molecular data and innovative trial frameworks, emerging models promise to enhance precision medicine’s impact and accelerate the delivery of personalized therapies to patients.
List of Major Active Trials by Phase, Sponsor, and Indication, A Key Example:
Clinical Trial Study Title
Trial Studying Chemotherapy in Nigerian Women With Triple Negative Breast Cancer
Study Status
Not Yet Recruiting
Phase
Phase II
Study Type
Interventional
Study Design
- Allocation: NA
- Intervention Model: SINGLE_GROUP
- Masking: NONE
- Primary Purpose: TREATMENT
Conditions
- Triple Negative Breast Cancer
Interventions
DRUG: Cyclophosphamide
DRUG: Epirubicin
DRUG: Docetaxel
DRUG: Carboplatin
PROCEDURE: Breast Surgery
DRUG: Capecitabine
Sponsor
University of Chicago
Number of Patients (Enrollment)
85
Start Date
1-11-2025
Primary Completion Date
3-04-2024
Completion Date
1-06-2032
Locations
Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Osun, 220005, Nigeria
Other aspects that shall be analyzed will include the market overview, clinical trials by study design, by key indications, by region, and list of key clinical trials, sponsors, among several other factors.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


