- Home
- »
- Market Trend Reports
- »
-
Tirzepatide (Mounjaro): Novel Solution For The Management Of Type 2 Diabetes And Obesity
Overview
The Tirzepatide (Mounjaro) market is experiencing notable developments, influenced by evolving regulatory standards, increasing demand, and shifting competitive dynamics within the pharmaceutical industry. As the market expands, it is essential to consider factors impacting its growth trajectory, emerging trends, and strategies employed by competitors. This report provides a detailed analysis of current market conditions, competitive positioning, and potential growth opportunities, delivering key insights to support strategic business decisions and investment opportunities within the pharmaceutical sector.
Key Report Deliverables
-
Analyze the Tirzepatide (Mounjaro) market landscape, detailing its current size, growth drivers, and key industry trends shaping the pharmaceutical sector.
-
Evaluate the competitive environment, identifying key players, their strategic moves, and the distribution of market share to understand competitive positioning.
-
Forecast market growth by projecting future trends, highlighting emerging opportunities, and assessing potential risks to growth.
-
Identify regulatory and market barriers, providing insights into challenges that could impact future market expansion and product development.
-
Concurrent Competitive Landscape, Assessing the current competitive environment, examining both direct and indirect competitors within the market.
Current Market Scenarios
The Tirzepatide (Mounjaro) market is emerging in response to the global health challenges associated with metabolic disorders, particularly type 2 diabetes and obesity. These conditions are widespread in developed markets such as the U.S., but their prevalence is also increasing in developing economies due to urbanization and lifestyle changes. With over 589 million people affected by diabetes worldwide, the demand for effective, patient-compliant therapies is substantial and continues to grow. Tirzepatide (Mounjaro)’s injectable formulation offers a viable solution in a market seeking more effective treatments, especially as other therapies face challenges related to patient adherence.
Clinical data for Tirzepatide (Mounjaro) has been promising, with results from the SURPASS clinical trials demonstrating significant reductions in HbA1c and body weight in patients with type 2 diabetes. These results highlight Tirzepatide (Mounjaro)’s potential to not only address the core metabolic issues but also provide a clinically meaningful benefit to patients by improving overall health outcomes.

With the global GLP-1 agonists weight loss drugs market valued at USD 13.84 billion in 2024 and projected to reach USD 48.84 billion by 2030, growing at a CAGR of 18.54%, Tirzepatide (Mounjaro) is strategically positioned to capture a significant share of this rapidly expanding segment. Its potential for growth is particularly high in emerging markets, where treatment access is increasing, reshaping the landscape of diabetes and obesity management.
Market Dynamics
The Power of Two: Tirzepatide’s Dual Action Mechanism
Tirzepatide (Mounjaro) operates through a dual-action mechanism that differentiates it from conventional diabetes and weight-loss treatments. By targeting both GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide), Tirzepatide addresses two key pathways that are critical in managing type 2 diabetes and promoting weight loss.
-
GLP-1 Agonism: GLP-1 plays a crucial role in insulin secretion, appetite regulation, and slowing gastric emptying. By mimicking GLP-1, Tirzepatide enhances insulin sensitivity, lowers blood sugar levels, and helps curb appetite, contributing to effective weight management.
-
GIP Agonism: GIP, on the other hand, directly influences insulin secretion in response to meals and plays a role in fat metabolism. Tirzepatide’s GIP receptor activation promotes better glucose control while also supporting fat breakdown, further amplifying its weight-loss potential.
This combination of GLP-1 and GIP allows Tirzepatide to provide a more comprehensive therapeutic approach than single-target therapies, such as semaglutide, which only activates the GLP-1 receptor. The dual mechanism results in enhanced efficacy for both blood sugar control and weight loss, offering a significant advantage for patients who may be managing both conditions simultaneously.

Side Effects and Risks Associated with Tirzepatide
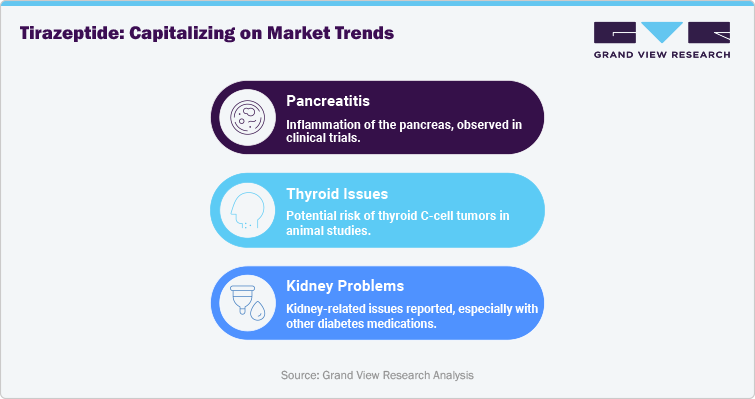
Tirzepatide is associated with several serious risks, including pancreatitis, thyroid issues, and kidney problems. Pancreatitis, or inflammation of the pancreas, has been observed in some clinical trials, particularly in patients with a history of pancreatic conditions. Animal studies have suggested a potential risk of thyroid C-cell tumors, though this has not been confirmed in humans. Tirzepatide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. Additionally, some patients have reported kidney-related issues, particularly when used alongside other diabetes medications that affect renal function. Healthcare providers must carefully screen patients for these underlying conditions and monitor them closely during treatment.
The more common side effects of Tirzepatide are primarily gastrointestinal. These include nausea, diarrhea, vomiting, and decreased appetite. While generally mild to moderate in nature, these effects are most experienced during the first few weeks of treatment as patients' bodies adjust. Nausea and diarrhea are particularly prevalent in the initial stages, while decreased appetite is often a beneficial side effect that contributes to weight loss, though it may be troublesome for some patients. These side effects can influence patient adherence, making it essential for healthcare providers to offer guidance on managing them to promote long-term treatment success.
“Capitalizing on Global Demand for Diabetes and Obesity Treatment”
Eli Lilly’s expansion strategy for Tirzepatide focuses not only on regulatory approvals but also on increasing manufacturing capacity to meet the growing demand for the drug. With a $5 billion investment in manufacturing infrastructure in the U.S., Eli Lilly is positioning Tirzepatide to meet the increasing demand, especially in developed markets. This move ensures that the company can effectively manage the supply-demand balance, enabling long-term growth and commercial success across multiple regions.
Tirzepatide’s dual-action mechanism, targeting both glycemic control and weight loss, presents a unique advantage in the treatment of type 2 diabetes and obesity. This dual-purpose nature positions Tirzepatide as a highly competitive option in global markets where the need for effective treatments for metabolic disorders is growing. As diabetes and obesity rates continue to rise globally, particularly in emerging markets like India and China, Tirzepatide’s ability to address both conditions makes it an appealing treatment option. These markets represent substantial growth opportunities, with increasing demand for accessible and effective therapies. Furthermore, Tirzepatide’s expanding global footprint and growing body of clinical evidence solidify its place as a significant player in the global healthcare market, ready to capture an increasing share of the obesity market, which is projected to grow significantly in the coming years.

“Mounjaro Market Thrives with Global Expansion”
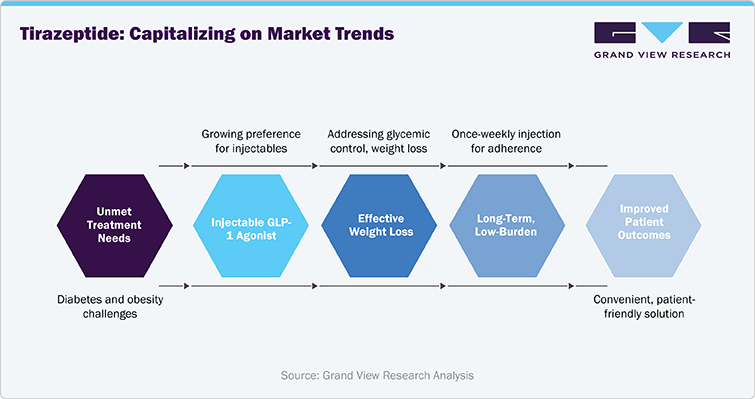
- Growing Preference for Injectable GLP-1 Agonists:
Tirzepatide, as a dual-action GLP-1 and GIP receptor agonist, is tapping into the increasing trend of adopting injectable GLP-1 agonists for diabetes and obesity management. As patients and healthcare providers recognize the efficacy of GLP-1-based therapies, Tirzepatide's unique mechanism is gaining traction, positioning it to capitalize on the growing acceptance of injectable treatments for metabolic disorders.
- Increased Demand for Weight Loss Solutions:
With obesity rates rising globally, there is a growing demand for effective weight loss treatments. Tirzepatide’s dual role in addressing both glycemic control and weight loss makes it well-positioned to benefit from this trend. As more patients with type 2 diabetes seek solutions for weight management, Tirzepatide’s proven efficacy in reducing body weight is becoming a key driver in its market adoption.
- Shift Toward Long-Term, Low-Burden Treatment Regimens:
The trend of patients seeking long-term, low-burden treatments is gaining momentum, and Tirzepatide’s once-weekly injection fits perfectly into this shift. Unlike daily injectables or frequent oral medications, the ease of a weekly regimen enhances patient adherence, reducing the treatment burden and supporting better long-term outcomes for patients. This trend supports Tirzepatide’s positioning as a convenient, patient-friendly solution in the competitive landscape of diabetes and obesity therapies.
Overview of Alternative Therapeutics
Alternative therapeutics for diabetes and obesity include a range of treatments such as GLP-1 receptor agonists (e.g., semaglutide and liraglutide), which offer benefits in glycemic control and weight loss, though some require daily administration compared to Tirzepatide’s once-weekly injection. SGLT-2 inhibitors (e.g., empagliflozin) offer oral alternatives that lower blood sugar but do not significantly aid in weight loss. Insulin therapy and metformin remain common for managing type 2 diabetes but are less effective in weight reduction. For more severe cases, bariatric surgery provides significant weight loss, though with higher risks and recovery time. Additionally, oral weight loss medications like phentermine-topiramate may help with appetite control but lack the dual benefits of glycemic control and weight loss seen in GLP-1 therapies. Tirzepatide stands out due to its dual-action mechanism and once-weekly dosing, offering a more convenient and comprehensive solution for managing both conditions.

Competitive Landscape
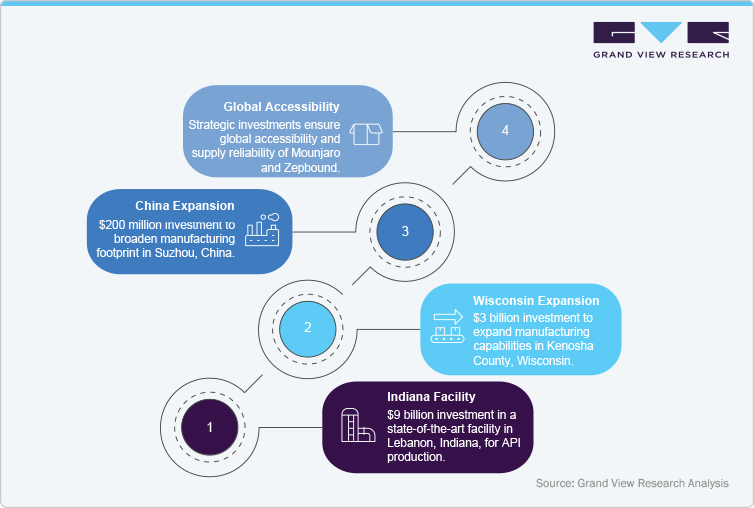
Eli Lilly and Company, the sole authorized manufacturer of Tirzepatide (Mounjaro), has significantly expanded its global manufacturing capabilities to meet the increasing demand for this dual GIP/GLP-1 receptor agonist. Recognizing the urgent need for treatments addressing type 2 diabetes and obesity, Lilly has committed over $23 billion since 2020 to enhance its production infrastructure across the United States and internationally.
A cornerstone of this expansion is the $9 billion investment in a state-of-the-art facility in Lebanon, Indiana, dedicated to producing the active pharmaceutical ingredient (API) for Tirzepatide (Mounjaro) and its obesity counterpart, Zepbound. This facility, expected to commence operations in 2026, represents the largest investment in synthetic medicine API manufacturing in U.S. history.
In addition to the Indiana site, Lilly is investing $3 billion to expand its manufacturing capabilities in Kenosha County, Wisconsin, aiming to bolster production of injectable treatments like Tirzepatide (Mounjaro) and Zepbound. This expansion is part of a broader initiative to enhance production capacity and ensure a steady supply of these critical medications.
Internationally, Lilly is broadening its manufacturing footprint with a $200 million expansion at its facility in Suzhou, China, to support the production of Tirzepatide (Mounjaro) and Zepbound. This move is aligned with Lilly's strategy to meet the growing demand for these treatments in emerging markets.
These strategic investments underscore Eli Lilly's commitment to scaling production of Tirzepatide (Mounjaro) and Zepbound, ensuring global accessibility and supply reliability. By strengthening its manufacturing infrastructure, Lilly aims to address the increasing global burden of type 2 diabetes and obesity, positioning Tirzepatide (Mounjaro) as a leading therapeutic option in the treatment landscape.
Analyst Perspective
“Tirzepatide (Mounjaro)'s dual-action mechanism and once-weekly dosing give it a competitive edge in the diabetes and obesity treatment landscape, positioning it for strong growth in both developed and emerging markets. Eli Lilly’s continued investment in manufacturing capacity ensures that the drug can meet increasing global demand, further solidifying its potential for long-term success.”

Case Study (Recent Engagement): GLP-1 Receptor Agonist Market Opportunity Assessment
Project Objective
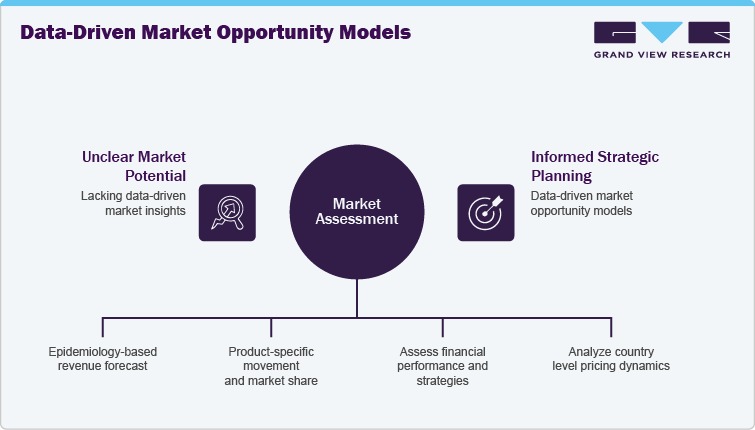
A leading global life sciences client approached us to assess the market potential and commercialization strategy for GLP-1 receptor agonist therapies across type 2 diabetes and obesity indications. The project aimed to support strategic planning for a novel, oral GLP-1 pipeline candidate, with a focus on launch timing, competitive positioning, and regional expansion.
GVR Solution
-
Conducted an epidemiology-based revenue forecast (2021-2036) using patient flow and analogue modeling approaches across North America, Europe, Asia Pacific, and the Middle East.
-
Delivered product-specific movement and market share analysis for:
-
Tirzepatide (Mounjaro) - used as a reference analogue for uptake modeling
-
Orforglipron (pipeline) - projected using analogue-based scenarios from comparable oral GLP-1 launches
-
-
Benchmarked key players such as Eli Lilly and Novo Nordisk across financial performance, product pipeline, and global rollout strategies.
-
Assessed country-level pricing, regulatory, and reimbursement dynamics, supported by a custom launch timeline and uptake forecast for Orforglipron, modeled analogously to prior GLP-1 innovations.
-
Provided outputs (Excel, PPT, dashboard) and ongoing strategic support tailored to the client’s internal planning and commercialization team needs.
Impact for Client
-
Created market models for launch planning and portfolio prioritization.
-
Guided product strategy with pricing, uptake, and competitor insights.
-
Identified growth markets and shaped regulatory and launch plans.
Why this Matters
-
Build analogue-based forecasts for emerging therapies
-
Provide product insights for pipeline drugs with no historical sales
-
Offer strategic guidance on market entry, launch, and clinical-commercial integration
We bring the same level of analytical rigor, therapeutic market expertise, and consultative flexibility to your assessment of the pancreatic cancer microbubble-based therapy market.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


