- Home
- »
- Clinical Diagnostics
- »
-
Clinical Laboratory Services Market Size, Share Report, 2030GVR Report cover
![Clinical Laboratory Services Market Size, Share & Trends Report]()
Clinical Laboratory Services Market Size, Share & Trends Analysis Report By Test Type (Human & Tumor Genetics, Clinical Chemistry), By Service Provider, By Application, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-250-1
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Clinical Laboratory Services Market Trends
The global clinical laboratory services market size was estimated at USD 233.24 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 3.5% from 2024 to 2030. The market growth is due to the factors such as increasing burden of chronic diseases and growing demand for early diagnostic tests. Moreover, rapid advancements in data management and sample preparation due to growing volumes of testing samples are anticipated to boost market growth during the forecast period.
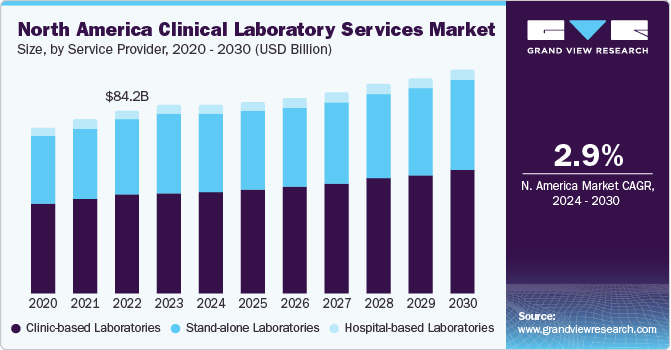
Clinical lab services are widely used for variety of applications, primarily for detecting and quantifying different biological substances. Variations in biomolecular concentrations can indicate various abnormal metabolic activities, infections, infectious and non-infectious diseases, and inflammatory conditions. To ensure optimal clinical results and overall public health, it's vital to rapidly and accurately diagnose severe illnesses and administer appropriate treatment.
Automation in clinical settings has significantly improved the data management process in laboratories. Growing adoption of laboratory automation systems is expected to boost the market during the forecast period. Furthermore, database management tools, patient test records, and integrated workflow management systems have received significant consideration in the healthcare industry. This can be attributed mainly to companies that are involved in processing nearly 100 to 150 billion samples per year. Hence, improvement & implementation of informatics and automated data management solutions to perform seamless operations are anticipated to drive the market.
An increase in disease prevalence and high demand for early disease diagnosis are expected to boost the market during the forecast period. These factors have prompted market players to introduce innovative services to address the growing demand. The introduction of accurate and technologically advanced products, such as companion diagnostics, biochips, & microarrays, has increased the demand for early disease detection. Products such as companion diagnostics also reduce the overall healthcare cost by helping physicians identify the most suitable treatment option based on the genetic makeup of a patient.Furthermore, growing prevalence of target diseases, such as cardiovascular diseases and diabetes, is a high-impact rendering driver of the market over the forecast period. Cardiovascular disease has become the most dominant cause of mortality and morbidity in the world in the past three decades. According to WHO, by 2030, cardiovascular diseases are estimated to cause approximately 23.6 million deaths, mainly due to heart disease and stroke. Moreover, the global prevalence of diabetes is constantly increasing, creating a large patient pool for the global market.
Market Concentration & Characteristics
Market growth stage is moderate, and pace of the market growth is accelerating. The market is characterized by a moderate degree of innovation. This can be attributed to the advanced technologies and methodologies for transforming diagnostic practices. From novel testing approaches to automation, the sector continually evolves, enhancing accuracy and efficiency in healthcare diagnostics. For instance, in January 2023, QIAGEN released EZ2 Connect MDx, an in-vitro diagnostics platform for automated sample processing for diagnostic laboratories. The device allows labs to purify RNA and DNA samples in 30 minutes.
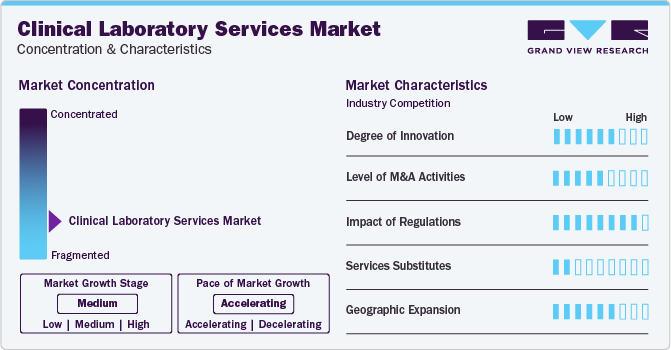
The market witnesses a notable M&A activities by the leading players. Leading players are strategically joining forces toexpand and enhance their services, gain access to new technologies, consolidate in the rapidly growing market, and address the increasing strategic importance of clinical laboratory services. In February 2022, Laboratory Corporation of America Holdings acquired Personal Genome Diagnostics Inc. to expand its portfolio of oncology-based NGS services.
The clinical laboratory services market is also subject to increasing regulatory scrutiny. The regulations ensure ensure safety standards, quality control, and fair competition. Countries have designated regulatory bodies that regulate the clinical laboratory services.In the United States, the application and safety aspects of laboratory tests and services are regulated and reviewed by the federal government through the Clinical Laboratory Improvement Amendments (CLIA). In addition to CLIA, the U.S. FDA is also involved in the regulations for the market.
There are limited direct substitutes for clinical laboratory services, such as at-home diagnostics kits and direct-to-consumer lab testing. However, these substitutes often lack the same level of performance and flexibility, emphasizing the unique value proposition of advanced clinical laboratory services.
The market is witnessing a growing number of geographical expansion strategies, with key players such as Quest Diagnostics, and LabCorp, employing this approach to broaden their service reach. This strategy enhances service availability across diverse geographic areas, ensuring a more extensive market presence.
Test Type Insights
Based on test type, the clinical chemistry segment led the market with the largest revenue share of 56.6% in 2023. The dominance of the segment is attributed to the presence of numerous clinical chemistry tests involved in pathology analysis of body fluids, including analysis of urine, plasma, serum, and other body fluids. Clinical chemistry tests or biochemistry tests form an integral part of basic level diagnosis and laboratory testing. Some of the common tests for clinical chemistry include glucose, creatinine, urea, total protein, globulins, albumin, bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), andgamma-glutamyl transpeptidase (GGT) among others. Techniques such as spectrophotometry, immunoassay, and electrophoresis are used to measure the concentration of different types of molecules present in the collected sample. Increasing automation to enhance customer experience is gaining traction in the segment.
The genetics testing segment is anticipated to witness the lucrative CAGR during the forecast period. This can be attributed to an increase in intensive research activities on genetic and proteomic studies, in context to hereditary & gene-mutation-related disorders. Moreover, there is an increasing demand for personalized care with accurate and early diagnosis in oncology is expected to fuel segment growth. Rising need for efficient tests in early diagnosis of major infections and cancer is anticipated to boost the segment growth.
Service Provider Insights
Based on the service provider, The hospital-based laboratories segment led the market in 2023 with a largest revenue share of 53.7%. This dominance is attributed to the high turnaround number of patients’ tests majorly for complex and severe disease conditions that are comparatively more cost-intensive. It is expected to maintain its dominance owing to the increasing number of hospitals integrating laboratories into their premises. The expansion of outreach programs initiated by hospitals, along with the quick turnaround of patients grappling with severe and major diseases, is projected to propel the segment growth. For instance, in February 2023, Tenaris along with San Jose Municipal Hospital in Campana, launched a new laboratory in Argentina to improve the capability of testing in the country.
The stand-alone laboratories segment is anticiapated to witness a lucrative CAGR over the forecast period. The market growth is owing to efforts to improve patient outcomes by providing diagnostic facilities at the retail level. Moreover, the ability of standalone labs to handle large volumes of diagnostic tests at an expedited rate and provide better results at comparatively lower prices is anticipated to offer economies of scale to service providers. There has been an increasing growth of standalone market since the outbreak of COVID-19 pandemic. In addition, emerging players providing standalone clinical lab testing services are expected to make a significant contribution to the segment’s rapid growth.
Application Insights
Based on application, the bioanalytical & lab chemistry services segment held the largest market revenue share of 52.1% in 2023. Bioanalytical & lab chemistry laboratories use a wide range of techniques and technology platforms to fulfill diagnostic needs. ELISA, chromatography, immunochemistry, mass spectroscopy, and molecular biology, are the most commonly used technologies in bioanalytical & lab chemistry applications. Bioanalytical services are an essential tool in drug discovery and development for determining the concentration of drugs and their metabolites. For instance, in November 2021, Labcorp launched a bioanalytical laboratory in Singapore expanding its bioanalytical presence in the Asia Pacific.
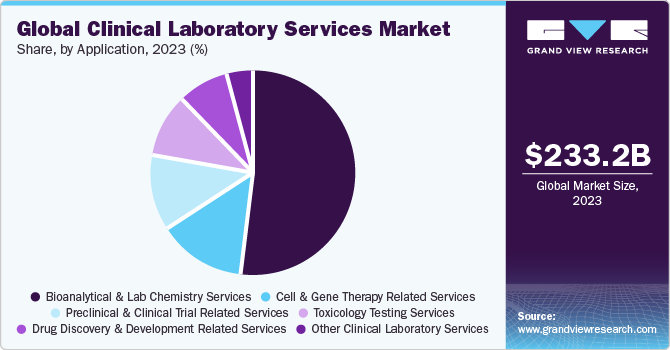
The toxicology testing services segment is expected to register a lucrative CAGR during the forecast period. Toxicology testing services include the identification of chemicals, drugs, and other toxic elements that affect patients and help clinicians in predicting future toxic effects, confirming a different diagnosis, or guiding a therapy. Moreover, market players are adopting various strategies, including mergers and acquisitions, contributing to segment growth. For instance, in January 2023, Aegis Sciences Corporation acquired HealthTrackRx's Toxicology business unit, strengthening Aegis's toxicology portfolio. This allows Aegis to provide comprehensive clinical toxicology testing, while HealthTrackRx concentrates on expanding its capabilities in PCR infectious disease testing. Similarly, in August 2022, LifeNet Health LifeSciences launched a human-relevant cell-based assay service by the acquisition of IONTOX, aiding in cytotoxic screening, biocompatibility assay, and a next-generation multi-organ platform. The main services in toxicity testing include urine testing, hair testing, blood testing, and saliva testing.
Regional Insights
North America dominated the market with a revenue share of 37.24% in 2023. Major factors contributing to the largest share include high prevalence of chronic diseases, well established healthcare infrastructure, and rising geriatric population. The region shows presence of well established healthcare system and reimbursement framework for clinical laboratory services. Moreover, new players are entering the market owing to the lucrative nature of the business in the country. For instance, in February 2023, Dutch biotech company Detact Diagnostics announced setting up a new laboratory in the Keene State College (U.S.) with a 2-year rental contract. Moreover, leading clinical laboratories that offer patients and healthcare professionals with crucial diagnostic information are represented by the American Clinical Laboratory, a national trade group.
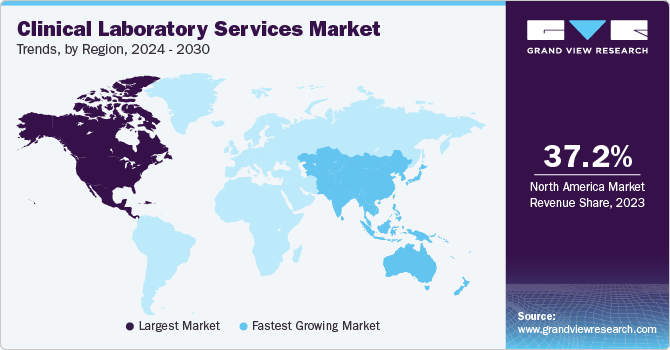
Asia Pacific. is anticipated to witness the fastest CAGR over the forecast period. The growth of the region is largely due to escalating scientific research, unmet medical needs, economic growth, and improvements in healthcare infrastructure and regulations. Increasing public awareness, and the availability of advanced medical treatments are anticipated to bolster the region's growth. Clinical laboratories serivces have gained significant importance during the COVID-19 pandemic due to heightened demand for testing. Major players are focusing on partnerships to introduce new lab testing services.
Key Clinical Laboratory Services Company Insights
Some of the key players operating in the market are Laboratory Corporation of America Holdings, Quest Diagnostics Incorporated, QIAGEN NV, Eurofins Scientific SE, Siemens Medical Solutions USA, Inc., and NeoGenomics Laboratories. These key players adopt various strategies to compete with their peers and drive growth. Companies are involved in expanding their market presence by entering into partnerships, collaborations, and agreements with other players to expand their product portfolio and geographic reach.
OPKO Health, Inc., ARUP Laboratories,and Sonic Healthcare are some of the emerging market participants in the global market. Emerging companies are employing various strategies to expand their footprint and grow at a fast pace. Customer acquisition strategies are being adopted by partnering with hospitals and clinics.
Key Clinical Laboratory Services Companies:
The following are the leading companies in the clinical laboratory services market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these clinical laboratory services companies are analyzed to map the supply network.
- Laboratory Corporation of America Holdings (LabCorp)
- QIAGEN NV
- Eurofins Scientific SE
- Quest Diagnostics Incorporated
- OPKO Health, Inc.
- Siemens Medical Solutions USA, Inc.
- NeoGenomics Laboratories
- Fresenius Medical Care
- ARUP Laboratories
- Sonic Healthcare
- Charles River Laboratories International, Inc.
- SYNLAB International GmbH
- Mayo Clinic Laboratories
- Unilabs
Recent Developments
- In August 2023, Laboratory Corporation of America Holdings entered into an agreement with Tufts Medicine. Under the agreement, Labcorp will acquire Tufts Medicine Outreach Laboratory Business to expand its diagnostic testing and laboratory services
- In July 2023, DiaCarta, Ltd. partnered with Hopkins MedTech Lab Services and Hopkins MedTech Compliance to support development and validation of laboratory developed tests in the U.S.
- In February 2023, Dutch biotech company Detact Diagnostics announced setting up a new laboratory in the Keene State College (U.S.) with a 2-year rental contract
Clinical Laboratory Service Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 233.63 billion
Revenue forecast in 2030
USD 286.77 billion
Growth rate
CAGR of 3.5% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion/million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Test type, application, service provider, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark, Sweden, Norway, China; Japan; India; Australia; South Korea; Brazil; Mexico; Colombia, Peru, Argentina; South Africa; Saudi Arabia; UAE;
Key companies profiled
Laboratory Corporation of America Holdings (LabCorp); QIAGEN NV; Eurofins Scientific SE; Quest Diagnostics Incorporated; OPKO Health, Inc; Siemens Medical Solutions USA, Inc.; NeoGenomics Laboratories; Fresenius Medical Care; ARUP Laboratories; Sonic Healthcare; Charles River Laboratories International, Inc; SYNLAB International GmbH; Mayo Clinic Laboratories; Unilabs
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase option
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Clinical Laboratory Services Market Report Segmentation
This report forecasts revenue growth at global, regional and country levels and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the clinical laboratory services market on thetest type,application, service provider, and region:
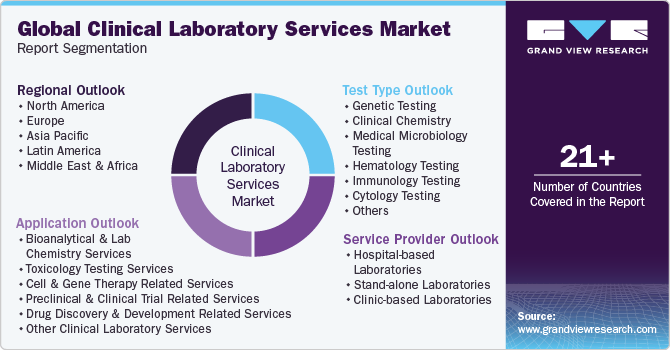
-
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Genetic Testing
-
Clinical Chemistry
-
Routine Chemistry Testing
-
Therapeutic Drug Monitoring Testing
-
Endocrinology Chemistry Testing
-
Specialized Chemistry Testing
-
Other Clinical Chemistry Testing
-
-
Medical Microbiology Testing
-
Infectious Disease Testing
-
Transplant Diagnostic Testing
-
Other Microbiology Testing
-
-
Hematology Testing
-
Immunology Testing
-
Cytology Testing
-
Drug of Abuse Testing
-
Other Esoteric Tests
-
-
Service Provider Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital-based Laboratories
-
Stand-alone Laboratories
-
Clinic-based Laboratories
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Bioanalytical & Lab Chemistry Services
-
Toxicology Testing Services
-
Cell & Gene Therapy Related Services
-
Preclinical & Clinical Trial Related Services
-
Drug Discovery & Development Related Services
-
Other Clinical Laboratory Services
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Colombia
-
Peru
-
Argentina
-
-
Middle East and Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global clinical laboratory service market size was estimated at USD 233.24 billion in 2023 and is expected to reach USD 233.63 billion in 2024.
b. The global clinical laboratory service market is expected to grow at a compound annual growth rate of 3.47% from 2024 to 2030 to reach USD 286.77 billion by 2030.
b. Clinical chemistry dominated the clinical laboratory service market with a share of 56.60% in 2023 owing to the wide usage of tests related to urine, plasma, serum, and other body fluids for disease screening.
b. Some of the key service providers in the market include QIAGEN, ARUP Laboratories., Quest Diagnostics Incorporated, OPKO Health, Inc., Abbott, Siemens Medical Solutions USA, Inc., NeoGenomics Laboratories., Fresenius Medical Care., Sonic Healthcare., Laboratory Corporation of America Holdings (LabCorp).
b. Key factors that are driving the clinical laboratory service market growth include the increasing burden of chronic disease prevalence coupled with consequent demand for the early diagnostic tests and rapid advances in data management & sample preparation due to growing volumes of testing samples.
b. North America led the clinical laboratory service market for clinical laboratory services and accounted for the largest revenue share of 37.24% in 2023, owing to the high burden of chronic diseases in the region.
Table of Contents
Chapter 1 Clinical Laboratory Services Market: Methodology And Scope
1.1 Market Segmentation
1.1.1 Segment Scope
1.1.2 Regional Scope
1.1.3 Estimates And Forecast Timeline
1.2 Research Methodology
1.3 Information Procurement
1.3.1 Purchased Database
1.3.2 Gvr’s Internal Database
1.3.3 Secondary Sources
1.3.4 Primary Research
1.3.5 Details Of Primary Research
1.4 Information Or Data Analysis
1.4.1 Data Analysis Models
1.5 Market Formulation & Validation
1.6 Model Details
1.6.1 Commodity Flow Analysis
1.6.1.1 Approach 1: Commodity Flow Approach
1.6.1.2 Approach 2: Country Wise Market Estimation Using Bottom Up Approach
1.6.1.3 Approach 2: Country Wise Market Estimation Using Top Down Approach
1.7 Global Market: Cagr Calculation
1.8 Global Market: Cagr Calculation
1.9 List Of Secondary Sources
1.10 Objectives
1.10.1 Objective 1:
1.10.2 Objective 2:
1.11 List Of Abbreviations
Chapter 2 Clinical Laboratory Services Market: Executive Summary
2.1 Clinical Laboratory Services Market: Market Outlook
2.1.1 Market Summary
Chapter 3 Clinical Laboratory Services Market: Industry Outlook
3.1 Market Lineage Outlook
3.1.1 Parent Market Lineage Outlook
3.1.2 Related/Ancillary Market Outlook
3.2 Penetration & Growth Prospect Mapping
3.3 Market Dynamics
3.3.1 Market Drivers
3.3.1.1 Technological Advancements In The Filed Of Clinical Testing
3.3.1.2 Growing Prevalence Of Target Diseases Coupled With Rising Demand For Early Disease Diagnostic Tests
3.3.1.3 Introduction Of Novel Solutions
3.3.1.4 Introduction Of Home Health Tests
3.3.1.5 Outbreak Of Coivd-19 Technologies
3.3.2 Market Restraint Analysis
3.3.2.1 Presence Of Stringent Regulatory Framework
3.4 Swot Analysis, By Factor (Political & Legal, Economic And Technological)
3.5 Industry Analysis - Porter’s
3.6 Reimbursement & Regulatory Scenario
3.7 Major Deals & Strategic Alliances
3.7.1 New Product Launch
3.7.2 Acquisition
3.7.3 Expansion
3.7.4 Partnerships
3.7.5 Marketing & Promotions
Chapter 4. Clinical Laboratory Services Market: Test Type Estimates & Trend Analysis
4.1. Test Type Market Share, 2023 & 2030
4.2. Segment Dashboard
4.3. Global Clinical Laboratory Services Market by Test Type Outlook
4.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
4.4.1. Genetic Testing
4.4.1.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.2. Clinical Chemistry
4.4.2.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.2.2. Routine Chemistry Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.2.3. Therapeutic Drug Monitoring Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.2.4. Endocrinology Chemistry Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.2.5. Specialized Chemistry Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.2.6. Other Clinical Chemistry Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.3. Medical Microbiology Testing
4.4.3.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.3.2. Infectious Disease Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.3.3. Transplant Diagnostic Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.3.4. Other Microbiology Testing Market estimates and forecasts, 2018 to 2030 (USD Million)
4.4.4. Hematology Testing
4.4.5. Immunology Testing
4.4.6. Cytology Testing
4.4.7. Drug of Abuse Testing
4.4.8. Other Esoteric Tests
4.4.8.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 5. Clinical Laboratory Services Market: Service Provider Estimates & Trend Analysis
5.1. Service Provider Market Share, 2023 & 2030
5.2. Segment Dashboard
5.3. Global Clinical Laboratory Services Market by Service Provider Outlook
5.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
5.4.1. Hospital-Based Laboratories
5.4.1.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
5.4.2. Stand-Alone Laboratories
5.4.2.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
5.4.3. Clinic-Based Laboratories
5.4.3.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 6. Clinical Laboratory Services Market: Application Estimates & Trend Analysis
6.1. Application Market Share, 2023 & 2030
6.2. Segment Dashboard
6.3. Global Clinical Laboratory Services Market by Application Outlook
6.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
6.4.1. Bioanalytical & Lab Chemistry Services
6.4.1.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.2. Toxicology Testing Services
6.4.2.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.3. Cell & Gene Therapy Related Services
6.4.3.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.4. Preclinical & Clinical Trial Related Services
6.4.4.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.5. Drug Discovery & Development Related Services
6.4.5.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
6.4.6. Others
6.4.6.1. Market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 7. Clinical Laboratory Services Market: Regional Estimates & Trend Analysis
7.1. Regional Market Share Analysis, 2023 & 2030
7.2. Regional Market Dashboard
7.3. Global Regional Market Snapshot
7.4. Market Size, & Forecasts Trend Analysis, 2018 to 2030
7.5. North America
7.5.1. U.S.
7.5.1.1. Key country dynamics
7.5.1.2. Regulatory framework/ reimbursement structure
7.5.1.3. Competitive scenario
7.5.1.4. U.S. market estimates and forecasts, 2018 to 2030 (USD Million)
7.5.2. Canada
7.5.2.1. Key country dynamics
7.5.2.2. Regulatory framework/ reimbursement structure
7.5.2.3. Competitive scenario
7.5.2.4. Canada market estimates and forecasts, 2018 to 2030 (USD Million)
7.6. Europe
7.6.1. UK
7.6.1.1. Key country dynamics
7.6.1.2. Regulatory framework/ reimbursement structure
7.6.1.3. Competitive scenario
7.6.1.4. UK market estimates and forecasts, 2018 to 2030 (USD Million)
7.6.2. Germany
7.6.2.1. Key country dynamics
7.6.2.2. Regulatory framework/ reimbursement structure
7.6.2.3. Competitive scenario
7.6.2.4. Germany market estimates and forecasts, 2018 to 2030 (USD Million)
7.6.3. France
7.6.3.1. Key country dynamics
7.6.3.2. Regulatory framework/ reimbursement structure
7.6.3.3. Competitive scenario
7.6.3.4. France market estimates and forecasts, 2018 to 2030 (USD Million)
7.6.4. Italy
7.6.4.1. Key country dynamics
7.6.4.2. Regulatory framework/ reimbursement structure
7.6.4.3. Competitive scenario
7.6.4.4. Italy market estimates and forecasts, 2018 to 2030 (USD Million)
7.6.5. Spain
7.6.5.1. Key country dynamics
7.6.5.2. Regulatory framework/ reimbursement structure
7.6.5.3. Competitive scenario
7.6.5.4. Spain market estimates and forecasts, 2018 to 2030 (USD Million)
7.6.6. Norway
7.6.6.1. Key country dynamics
7.6.6.2. Regulatory framework/ reimbursement structure
7.6.6.3. Competitive scenario
7.6.6.4. Norway market estimates and forecasts, 2018 to 2030 (USD Million)
7.6.7. Sweden
7.6.7.1. Key country dynamics
7.6.7.2. Regulatory framework/ reimbursement structure
7.6.7.3. Competitive scenario
7.6.7.4. Sweden market estimates and forecasts, 2018 to 2030 (USD Million)
7.6.8. Denmark
7.6.8.1. Key country dynamics
7.6.8.2. Regulatory framework/ reimbursement structure
7.6.8.3. Competitive scenario
7.6.8.4. Denmark market estimates and forecasts, 2018 to 2030 (USD Million)
7.7. Asia Pacific
7.7.1. Japan
7.7.1.1. Key country dynamics
7.7.1.2. Regulatory framework/ reimbursement structure
7.7.1.3. Competitive scenario
7.7.1.4. Japan market estimates and forecasts, 2018 to 2030 (USD Million)
7.7.2. China
7.7.2.1. Key country dynamics
7.7.2.2. Regulatory framework/ reimbursement structure
7.7.2.3. Competitive scenario
7.7.2.4. China market estimates and forecasts, 2018 to 2030 (USD Million)
7.7.3. India
7.7.3.1. Key country dynamics
7.7.3.2. Regulatory framework/ reimbursement structure
7.7.3.3. Competitive scenario
7.7.3.4. India market estimates and forecasts, 2018 to 2030 (USD Million)
7.7.4. Australia
7.7.4.1. Key country dynamics
7.7.4.2. Regulatory framework/ reimbursement structure
7.7.4.3. Competitive scenario
7.7.4.4. Australia market estimates and forecasts, 2018 to 2030 (USD Million)
7.7.5. South Korea
7.7.5.1. Key country dynamics
7.7.5.2. Regulatory framework/ reimbursement structure
7.7.5.3. Competitive scenario
7.7.5.4. South Korea market estimates and forecasts, 2018 to 2030 (USD Million)
7.7.6. Thailand
7.7.6.1. Key country dynamics
7.7.6.2. Regulatory framework/ reimbursement structure
7.7.6.3. Competitive scenario
7.7.6.4. Thailand market estimates and forecasts, 2018 to 2030 (USD Million)
7.8. Latin America
7.8.1. Brazil
7.8.1.1. Key country dynamics
7.8.1.2. Regulatory framework/ reimbursement structure
7.8.1.3. Competitive scenario
7.8.1.4. Brazil market estimates and forecasts, 2018 to 2030 (USD Million)
7.8.2. Mexico
7.8.2.1. Key country dynamics
7.8.2.2. Regulatory framework/ reimbursement structure
7.8.2.3. Competitive scenario
7.8.2.4. Mexico market estimates and forecasts, 2018 to 2030 (USD Million)
7.8.3. Argentina
7.8.3.1. Key country dynamics
7.8.3.2. Regulatory framework/ reimbursement structure
7.8.3.3. Competitive scenario
7.8.3.4. Argentina market estimates and forecasts, 2018 to 2030 (USD Million)
7.8.4. Colombia
7.8.4.1. Key country dynamics
7.8.4.2. Regulatory framework/ reimbursement structure
7.8.4.3. Competitive scenario
7.8.4.4. Colombia market estimates and forecasts, 2018 to 2030 (USD Million)
7.8.5. Peru
7.8.5.1. Key country dynamics
7.8.5.2. Regulatory framework/ reimbursement structure
7.8.5.3. Competitive scenario
7.8.5.4. Peru market estimates and forecasts, 2018 to 2030 (USD Million)
7.9. MEA
7.9.1. South Africa
7.9.1.1. Key country dynamics
7.9.1.2. Regulatory framework/ reimbursement structure
7.9.1.3. Competitive scenario
7.9.1.4. South Africa market estimates and forecasts, 2018 to 2030 (USD Million)
7.9.2. Saudi Arabia
7.9.2.1. Key country dynamics
7.9.2.2. Regulatory framework/ reimbursement structure
7.9.2.3. Competitive scenario
7.9.2.4. Saudi Arabia market estimates and forecasts, 2018 to 2030 (USD Million)
7.9.3. UAE
7.9.3.1. Key country dynamics
7.9.3.2. Regulatory framework/ reimbursement structure
7.9.3.3. Competitive scenario
7.9.3.4. UAE market estimates and forecasts, 2018 to 2030 (USD Million)
7.9.4. Kuwait
7.9.4.1. Key country dynamics
7.9.4.2. Regulatory framework/ reimbursement structure
7.9.4.3. Competitive scenario
7.9.4.4. Kuwait market estimates and forecasts, 2018 to 2030 (USD Million)
Chapter 8. Competitive Landscape
8.1. Recent Developments & Impact Analysis, By Key Market Participants
8.2. Company/Competition Categorization
8.3. Vendor Landscape
8.3.1. List of key distributors and channel partners
8.3.2. Key customers
8.3.3. Key company market share analysis, 2023
8.3.4. LABORATORY CORPORATION OF AMERICA HOLDINGS (LABCORP)
8.3.4.1. Company overview
8.3.4.2. Financial performance
8.3.4.3. Product benchmarking
8.3.4.4. Strategic initiatives
8.3.5. QIAGEN NV
8.3.5.1. Company overview
8.3.5.2. Financial performance
8.3.5.3. Product benchmarking
8.3.5.4. Strategic initiatives
8.3.6. QUEST DIAGNOSTICS INCORPORATED
8.3.6.1. Company overview
8.3.6.2. Financial performance
8.3.6.3. Product benchmarking
8.3.6.4. Strategic initiatives
8.3.7. OPKO HEALTH, INC
8.3.7.1. Company overview
8.3.7.2. Financial performance
8.3.7.3. Product benchmarking
8.3.7.4. Strategic initiatives
8.3.8. CHARLES RIVER LABORATORIES
8.3.8.1. Company overview
8.3.8.2. Financial performance
8.3.8.3. Product benchmarking
8.3.8.4. Strategic initiatives
8.3.9. ARUP LABORATORIES (ASSOCIATED REGIONAL AND UNIVERSITY PATHOLOGISTS, INC.)
8.3.9.1. Company overview
8.3.9.2. Financial performance
8.3.9.3. Product benchmarking
8.3.9.4. Strategic initiatives
8.3.10. SONIC HEALTHCARE
8.3.10.1. Company overview
8.3.10.2. Financial performance
8.3.10.3. Product benchmarking
8.3.10.4. Strategic initiatives
8.3.11. NEOGENOMICS LABORATORIES, INC.
8.3.11.1. Company overview
8.3.11.2. Financial performance
8.3.11.3. Product benchmarking
8.3.11.4. Strategic initiatives
8.3.12. FRESENIUS MEDICAL CARE AG & CO. KGAA
8.3.12.1. Company overview
8.3.12.2. Financial performance
8.3.12.3. Product benchmarking
8.3.12.4. Strategic initiatives
8.3.13. SIEMENS HEALTHCARE LIMITED
8.3.13.1. Company overview
8.3.13.2. Financial performance
8.3.13.3. Product benchmarking
8.3.13.4. Strategic initiatives
8.3.14. SYNLAB International GmbH
8.3.14.1. Company overview
8.3.14.2. Financial performance
8.3.14.3. Product benchmarking
8.3.14.4. Strategic initiatives
8.3.15. Mayo Clinic Laboratories
8.3.15.1. Company overview
8.3.15.2. Financial performance
8.3.15.3. Product benchmarking
8.3.15.4. Strategic initiatives
8.3.16. Unilabs
8.3.16.1. Company overview
8.3.16.2. Financial performance
8.3.16.3. Product benchmarking
8.3.16.4. Strategic initiatives
8.3.17. Eurofins Scientific SE
8.3.17.1. Company overview
8.3.17.2. Financial performance
8.3.17.3. Product benchmarking
8.3.17.4. Strategic initiatives
List of Tables
Table 1 List of Abbreviation
Table 2 North America clinical laboratory services market, by region, 2018 - 2030 (USD Million)
Table 3 North America clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 4 North America clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 5 North America clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 6 U.S. clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 7 U.S. clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 8 U.S. clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 9 Canada clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 10 Canada clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 11 Canada clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 12 Europe clinical laboratory services market, by region, 2018 - 2030 (USD Million)
Table 13 Europe clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 14 Europe clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 15 Europe clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 16 Germany clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 17 Germany clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 18 Germany clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 19 UK clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 20 UK clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 21 UK clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 22 France clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 23 France clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 24 France clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 25 Italy clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 26 Italy clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 27 Italy clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 28 Spain clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 29 Spain clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 30 Spain clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 31 Denmark clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 32 Denmark clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 33 Denmark clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 34 Sweden clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 35 Sweden clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 36 Sweden clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 37 Norway clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 38 Norway clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 39 Norway clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 40 Asia Pacific clinical laboratory services market, by region, 2018 - 2030 (USD Million)
Table 41 Asia Pacific clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 42 Asia Pacific clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 43 Asia Pacific clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 44 China clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 45 China clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 46 China clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 47 Japan clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 48 Japan clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 49 Japan clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 50 India clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 51 India clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 52 India clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 53 South Korea clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 54 South Korea clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 55 South Korea clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 56 Australia clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 57 Australia clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 58 Australia clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 59 Thailand clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 60 Thailand clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 61 Thailand clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 62 Latin America clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 63 Latin America clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 64 Latin America clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 65 Brazil clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 66 Brazil clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 67 Brazil clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 68 Mexico clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 69 Mexico clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 70 Mexico clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 71 Argentina clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 72 Argentina clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 73 Argentina clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 74 Colombia clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 75 Colombia clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 76 Colombia clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 77 Peru clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 78 Peru clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 79 Peru clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 80 MEA clinical laboratory services market, by region, 2018 - 2030 (USD Million)
Table 81 MEA clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 82 MEA clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 83 MEA clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 84 South Africa clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 85 South Africa clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 86 South Africa clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 87 Saudi Arabia clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 88 Saudi Arabia clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 89 Saudi Arabia clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 90 UAE clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 91 UAE clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 92 UAE clinical laboratory services market, by application, 2018 - 2030 (USD Million)
Table 93 Kuwait clinical laboratory services market, by test type, 2018 - 2030 (USD Million)
Table 94 Kuwait clinical laboratory services market, by service provider, 2018 - 2030 (USD Million)
Table 95 Kuwait clinical laboratory services market, by application, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Market research process
Fig. 2 Data triangulation techniques
Fig. 3 Primary research pattern
Fig. 4 Primary interviews in North America
Fig. 5 Primary interviews in Europe
Fig. 6 Primary interviews in APAC
Fig. 7 Primary interviews in Latin America
Fig. 8 Primary interviews in MEA
Fig. 9 Market research approaches
Fig. 10 Value-chain-based sizing & forecasting
Fig. 11 QFD modeling for market share assessment
Fig. 12 Market formulation & validation
Fig. 13 Clinical laboratory services market: market outlook
Fig. 14 Clinical laboratory services competitive insights
Fig. 15 Parent market outlook
Fig. 16 Related/ancillary market outlook
Fig. 17 Penetration and growth prospect mapping
Fig. 18 Industry value chain analysis
Fig. 19 Clinical laboratory services market driver impact
Fig. 20 Clinical laboratory services market restraint impact
Fig. 21 Clinical laboratory services market strategic initiatives analysis
Fig. 22 Clinical laboratory services market: Test Type movement analysis
Fig. 23 Clinical laboratory services market: Test Type outlook and key takeaways
Fig. 24 Genetic Testing market estimates and forecast, 2018 - 2030
Fig. 25 Clinical chemistry market estimates and forecast, 2018 - 2030
Fig. 26 Routine chemistry testing market estimates and forecast, 2018 - 2030
Fig. 27 Therapeutic drug monitoring testing market estimates and forecast, 2018 - 2030
Fig. 28 Endocrinology chemistry testing market estimates and forecast, 2018 - 2030
Fig. 29 Specialized chemistry testing market estimates and forecast, 2018 - 2030
Fig. 30 Other clinical chemistry testing market estimates and forecast, 2018 - 2030
Fig. 31 Medical microbiology testing market estimates and forecast, 2018 - 2030
Fig. 32 Infectious disease testing market estimates and forecast, 2018 - 2030
Fig. 33 Transplant diagnostic testing market estimates and forecast, 2018 - 2030
Fig. 34 Other microbiology testing market estimates and forecast, 2018 - 2030
Fig. 35 Hematology testing market estimates and forecast, 2018 - 2030
Fig. 36 Immunology testing market estimates and forecast, 2018 - 2030
Fig. 37 Cytology testing market estimates and forecast, 2018 - 2030
Fig. 38 Drug of abuse testing market estimates and forecast, 2018 - 2030
Fig. 39 Other esoteric tests market estimates and forecast, 2018 - 2030
Fig. 40 Clinical laboratory services market: Service Provider movement Analysis
Fig. 41 Clinical laboratory services market: Service Provider outlook and key takeaways
Fig. 42 Hospital-based laboratories market estimates and forecasts, 2018 - 2030
Fig. 43 Stand-alone laboratories market estimates and forecasts,2018 - 2030
Fig. 44 Clinic-based laboratories market estimates and forecasts,2018 - 2030
Fig. 45 Clinical laboratory services market: application movement analysis
Fig. 46 Fig. .46 Clinical laboratory services market: application outlook and key takeaways
Fig. 47 Bioanalytical & lab chemistry services market estimates and forecasts, 2018 - 2030
Fig. 48 Toxicology testing services market estimates and forecasts,2018 - 2030
Fig. 49 Cell & gene therapy related services market estimates and forecasts, 2018 - 2030
Fig. 50 Preclinical & clinical trial related services market estimates and forecasts,2018 - 2030
Fig. 51 Drug discovery & development related services market estimates and forecasts, 2018 - 2030
Fig. 52 Other clinical laboratory services market estimates and forecasts,2018 - 2030
Fig. 53 Global clinical laboratory services market: Regional movement analysis
Fig. 54 Global clinical laboratory services market: Regional outlook and key takeaways
Fig. 55 Global clinical laboratory services market share and leading players
Fig. 56 North America market share and leading players
Fig. 57 Europe market share and leading players
Fig. 58 Asia Pacific market share and leading players
Fig. 59 Latin America market share and leading players
Fig. 60 Middle East & Africa market share and leading players
Fig. 61 North America: SWOT
Fig. 62 Europe SWOT
Fig. 63 Asia Pacific SWOT
Fig. 64 Latin America SWOT
Fig. 65 MEA SWOT
Fig. 66 North America
Fig. 67 North America market estimates and forecasts, 2018 - 2030
Fig. 68 U.S.
Fig. 69 U.S. market estimates and forecasts, 2018 - 2030
Fig. 70 Canada
Fig. 71 Canada market estimates and forecasts, 2018 - 2030
Fig. 72 Europe
Fig. 73 Europe market estimates and forecasts, 2018 - 2030
Fig. 74 UK
Fig. 75 UK market estimates and forecasts, 2018 - 2030
Fig. 76 Germany
Fig. 77 Germany market estimates and forecasts, 2018 - 2030
Fig. 78 France
Fig. 79 France market estimates and forecasts, 2018 - 2030
Fig. 80 Italy
Fig. 81 Italy market estimates and forecasts, 2018 - 2030
Fig. 82 Spain
Fig. 83 Spain market estimates and forecasts, 2018 - 2030
Fig. 84 Denmark
Fig. 85 Denmark market estimates and forecasts, 2018 - 2030
Fig. 86 Sweden
Fig. 87 Sweden market estimates and forecasts, 2018 - 2030
Fig. 88 Norway
Fig. 89 Norway market estimates and forecasts, 2018 - 2030
Fig. 90 Asia Pacific
Fig. 91 Asia Pacific market estimates and forecasts, 2018 - 2030
Fig. 92 China
Fig. 93 China market estimates and forecasts, 2018 - 2030
Fig. 94 Japan
Fig. 95 Japan market estimates and forecasts, 2018 - 2030
Fig. 96 India
Fig. 97 India market estimates and forecasts, 2018 - 2030
Fig. 98 Thailand
Fig. 99 Thailand market estimates and forecasts, 2018 - 2030
Fig. 100 South Korea
Fig. 101 South Korea market estimates and forecasts, 2018 - 2030
Fig. 102 Australia
Fig. 103 Australia market estimates and forecasts, 2018 - 2030
Fig. 104 Latin America
Fig. 105 Latin America market estimates and forecasts, 2018 - 2030
Fig. 106 Brazil
Fig. 107 Brazil market estimates and forecasts, 2018 - 2030
Fig. 108 Mexico
Fig. 109 Mexico market estimates and forecasts, 2018 - 2030
Fig. 110 Colombia
Fig. 111 Colombia market estimates and forecasts, 2018 - 2030
Fig. 112 Peru
Fig. 113 Peru market estimates and forecasts, 2018 - 2030
Fig. 114 Argentina
Fig. 115 Argentina market estimates and forecasts, 2018 - 2030
Fig. 116 Middle East and Africa
Fig. 117 Middle East and Africa market estimates and forecasts, 2018 - 2030
Fig. 118 South Africa
Fig. 119 South Africa market estimates and forecasts, 2018 - 2030
Fig. 120. Saudi Arabia
Fig. 121 Saudi Arabia market estimates and forecasts, 2018 - 2030
Fig. 122UAE
Fig. 123 UAE market estimates and forecasts, 2018 - 2030
Fig. 124 Kuwait
Fig. 125 Kuwait market estimates and forecasts, 2018 - 2030
Fig. 126 Market share of key market players - Clinical laboratory services marketWhat questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Clinical Laboratory Services Test Type Outlook (Revenue, USD Million, 2018 - 2030)
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Clinical Laboratory Services Service Provider Outlook (Revenue, USD Million, 2018 - 2030)
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Clinical Laboratory Services Application Outlook (Revenue, USD Million, 2018 - 2030)
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Clinical Laboratory ServicesRegional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- North America Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- North America Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- North America Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- U.S.
- U.S. Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- U.S. Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- U.S. Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- U.S. Clinical Laboratory Services, By Test Type
- Canada
- Canada Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Canada Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Canada Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Canada Clinical Laboratory Services, By Test Type
- North America Clinical Laboratory Services, By Test Type
- Europe
- Europe Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Europe Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Europe Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- UK
- UK Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- UK Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- UK Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- UK Clinical Laboratory Services, By Test Type
- Germany
- Germany Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Germany Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Germanya Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Germany Clinical Laboratory Services, By Test Type
- France
- France Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- France Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- France Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- France Clinical Laboratory Services, By Test Type
- Italy
- Italy Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Italy Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Italy Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Italy Clinical Laboratory Services, By Test Type
- Spain
- Spain Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Spain Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Spain Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Spain Clinical Laboratory Services, By Test Type
- Denmark
- Denmark Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Denmark Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Denmark Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Denmark Clinical Laboratory Services, By Test Type
- Sweden
- Sweden Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Sweden Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Sweden Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Sweden Clinical Laboratory Services, By Test Type
- Norway
- Norway Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Norway Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Norway Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Norway Clinical Laboratory Services, By Test Type
- Europe Clinical Laboratory Services, By Test Type
- Asia Pacific
- Asia Pacific Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Asia Pacific Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Asia Pacific Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Japan
- Japan Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Japan Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Japan Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Japan Clinical Laboratory Services, By Test Type
- China
- China Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- China Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- China Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- China Clinical Laboratory Services, By Test Type
- India
- India Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- India Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- India Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- India Clinical Laboratory Services, By Test Type
- Australia
- Australia Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Australia Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Australia Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Australia Clinical Laboratory Services, By Test Type
- South Korea
- South Korea Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- South Korea Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- South Korea Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- South Korea Clinical Laboratory Services, By Test Type
- Thailand
- Thailand Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Thailand Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Thailand Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Thailand Clinical Laboratory Services, By Test Type
- Asia Pacific Clinical Laboratory Services, By Test Type
- Latin America
- Latin America Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Latin America Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Latin America Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Brazil
- Brazil Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Brazila Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Brazil Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Brazil Clinical Laboratory Services, By Test Type
- Mexico
- Mexico Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Mexico Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Mexico Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Mexico Clinical Laboratory Services, By Test Type
- Colombia
- Colombia Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Colombia Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Colombia Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Colombia Clinical Laboratory Services, By Test Type
- Peru
- Peru Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Peru Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Peru Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Peru Clinical Laboratory Services, By Test Type
- Argentina
- Argentina Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Argentina Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Argentina Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Argentina Clinical Laboratory Services, By Test Type
- Latin America Clinical Laboratory Services, By Test Type
- Middle East and Africa
- Middle East and Africa Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Middle East and Africa Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Middle East and Africa Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- South Africa
- South Africa Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- South Africa Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- South Africa Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- South Africa Clinical Laboratory Services, By Test Type
- Saudi Arabia
- Saudi Arabia Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Saudi Arabia Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Saudi Arabia Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Saudi Arabia Clinical Laboratory Services, By Test Type
- UAE
- UAE Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- UAE Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- UAE Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- UAE Clinical Laboratory Services, By Test Type
- Kuwait
- Kuwait Clinical Laboratory Services, By Test Type
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
- Kuwait Clinical Laboratory Services, By Service Provider
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
- Kuwait Clinical Laboratory Services, By Application
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
- Kuwait Clinical Laboratory Services, By Test Type
- Middle East and Africa Clinical Laboratory Services, By Test Type
- North America
Clinical Laboratory Service Market Dynamics
Drivers:
Technological Advancements in the Field of Clinical Testing
Automation in clinical settings has significantly improved the data management process in laboratories. The growing adoption of laboratory automation systems is expected to boost the market during the forecast period. Furthermore, database management tools, patient test records, and integrated workflow management systems have received significant consideration in the healthcare industry. This can be attributed mainly to companies that are involved in processing nearly 100 to 150 billion samples per year. Hence, improvement & implementation of informatics and automated data management solutions to perform seamless operations are anticipated to drive the market. Advancements in laboratory testing technology through incremental and breakthrough developments are high-impact rendering drivers of the market. Incremental changes are introduced to make the testing processes simpler and enhance their efficiency. The introduction of novel solutions to maximize efficiency and minimize errors is anticipated to further boost the market. Market players are engaged in introducing new services to meet the unmet needs of patients. For instance, in May 2022, Guardant Health in partnership with Vall d’Hebron Institute of Oncology launched the first health liquid biopsy testing service in Europe for oncology.
Growing Prevalence of Target Disease and Rising Demand for Early Diagnostic Tests
An increase in disease prevalence and high demand for early disease diagnosis are expected to boost the market in the coming years. These factors have prompted market players to introduce innovative services to address the growing demand. The introduction of accurate and technologically advanced products, such as companion diagnostics, biochips, & microarrays, has increased the demand for early disease detection. This is mainly due to increasing healthcare expenditure as a consequence of the growing prevalence of chronic diseases. For instance, biochips are used for preparing thousands of DNA, RNA, or protein samples, which can be processed at the same time on a single chip. Products such as companion diagnostics also reduce the overall healthcare cost by helping physicians identify the most suitable treatment option based on the genetic makeup of a patient. Furthermore, the growing prevalence of target diseases, such as cardiovascular diseases and diabetes, is a high-impact rendering driver of the market over the forecast period. The rise in the prevalence of multiple cancers and the increase in the need to provide efficient methods to detect them at early stages to enable timely & appropriate treatment are anticipated to drive the market. For instance, according to the International Agency for Research on Cancer (IARC), over 20.7 million new cancer cases are expected to be diagnosed in 2023. The need to develop diagnostic options that can detect cancer at an early stage, which can help improve disease management & reduce mortality, is likely to propel the overall market growth.
Restraints:
Presence of Stringent Regulatory Framework
The regulatory framework for approvals is a major restraint in the pharmaceutical, biotechnology, and medical technology industries. In the U.S., usage and safety profiles of laboratory tests & services are highly regulated and evaluated by the federal government through the Clinical Laboratory Improvement Amendments (CLIA). Three federal agencies are involved in CLIA: The FDA, CMS, and CDC. Unclear regulatory frameworks/guidelines for diagnostics in developing economies are anticipated to restrain market growth. The presence of such discrete and uncertain scenarios in regulations for molecular diagnostic products can create confusion among manufacturers regarding commercialization. In addition, the lack of a robust reimbursement framework is expected to restrain market growth by limiting the purchasing power of buyers. However, there is a clear regulatory framework for IVD tests for diagnosis of diseases such as COVID-19 and the products are granted Emergency Use Authorization (EUA). For instance, in the U.S., products are approved under Section 564 of the Federal Food, Drug, and Cosmetic Act. Many challenges are likely to arise once this section is revoked and the products have to undergo regular approval for future use when the risk of COVID-19 infection decreases.
What Does This Report Include?
This section will provide insights into the contents included in this clinical laboratory service market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Clinical laboratory service market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Clinical laboratory service market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the clinical laboratory service market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for clinical laboratory service market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of clinical laboratory service market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Clinical Laboratory Service Market Categorization:
The clinical laboratory service market was categorized into four segments, namely test type (Human & Tumor Genetics, Clinical Chemistry, Medical Microbiology & Cytology), service provider (Hospital-based Laboratories, Stand-alone Laboratories, Clinic-based Laboratories), application (Bioanalytical & Lab Chemistry Services, Toxicology Testing Services, Cell & Gene Therapy Related Services, Preclinical & Clinical Trial Related Services, Drug Discovery & Development Related Services) region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa)
Segment Market Methodology:
The clinical laboratory service market was segmented into test type, service provider, application, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The clinical laboratory service market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into twenty namely, the U.S.; Canada; Germany; the UK.; France; Italy; Spain; China; Japan; India; Australia; South Korea; Brazil; Mexico; Colombia; Peru; Argentina; South Africa; Saudi Arabia; UAE,
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Clinical laboratory Service market companies & financials:
The clinical laboratory service market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
Abbott - Abbott has a presence in more than 150 countries with research, manufacturing, sales, and distribution facilities. Its pharmaceuticals segment offers generic medicines for pain, fever, migraine, inflammation, pancreatic disorders, & hypertension; cardiovascular & metabolic products; and vaccines. The diagnostic segment offers reagents and tests for various applications. The company’s diagnostics portfolio includes technologies such as polymerase chain reaction, fluorescence in situ hybridization, and DNA & RNA sequencing.
-
QIAGEN - QIAGEN is a company that caters to the biotechnology industry. It provides samples and assay technologies for academic research, applied testing, molecular diagnostics, & pharmaceutical research. The company markets over 500 products, including consumable kits and automation systems. Its products for molecular diagnostics cover relevant areas of healthcare, ranging from early diagnosis of diseases, establishment of diagnosis, and diagnostic testing for determination of suitable treatment to point-of-need testing. The company’s customers include healthcare providers, pharmaceutical & biotechnology companies, governments or industrial customers, and researchers.
-
Quest Diagnostics - Quest Diagnostics is a provider of clinical laboratory tests. The company was established as Metropolitan Pathology Laboratory, Inc., and in 1997 it became an independent corporation called Quest Diagnostics. The company has administrative offices in Lyndhurst, Collegeville, and Pennsylvania. In 2013, Fortune magazine ranked the company at apex position in pharmacy and other services. The company employs over 47,000 employees globally.
-
OPKO Health, Inc. - It is a healthcare company that established itself in large and growing markets. The company’s diagnostics business refers to BioReference Laboratories, a clinical lab with genetic testing ability that includes the 4Kscore, a prostate cancer screening test, and the Claros1 immunoassay testing platform. The company focuses on novel molecular diagnostics, point-of-care, vaccines, and proprietary pharmaceuticals. In August 2015, OPKO Health, Inc. acquired BioReference Laboratories.
-
Siemens Medical Solutions USA, Inc. - It is a provider of healthcare solutions such as diagnostics (in vitro & in vivo), imaging & therapy systems, clinical products, audiology solutions, and healthcare IT solutions. The company was founded as a separate entity in 2015. It also offers medical accessories, OEM component & medical electronics solutions, refurbished systems for medical imaging & therapy, and technical, maintenance, & professional & consulting services. Its products and solutions are used in various areas like therapeutic drug monitoring cardiology, organ transplant, urology, infectious diseases, diabetes, women's health, surgery, oncology, neurology, and growth disorders.
-
NeoGenomics Laboratories - In 2015, NeoGenomics acquired Clarient for USD 275 million. Clarient, Inc., formerly known as ChromaVision Medical Systems, was incorporated in 1993 and located in California, U.S. The company changed its name to Clarient, Inc. in 2005. The company is engaged in providing diagnostics and cancer testing services. It is focused on breast cancer recurrence testing, breast cancer testing, KRAS in colorectal cancer, colon cancer testing, pulmotype test, and mutation testing. The range of tests provided by the company includes FISH, BRAF, ALK Rearrangement, EGFR mutation analysis, Mammostrat, and PI3K.
-
Fresenius Medical Care - Fresenius Medical Care is a provider of products to treat chronic kidney failure. The company has worldwide operations supported by 37 production sites and offers dialysis products like dialysis machines & other related disposables. The company has its North American headquarters in Waltham, Massachusetts, and Asia Pacific headquarters in Hong Kong. In 1997, Fresenius Medical Care Holdings, Inc. acquired Spectra Laboratories for its kidney dialysis laboratory in the U.S.
-
ARUP Laboratories - Associated Regional and University Pathologists, Inc. is a nonprofit enterprise laboratory of the Department of Pathology, University of Utah. The company serves as the national reference for the U.S. ARUP provides a variety of about 3,000 tests and their combinations encompassing various fields of clinical medicine, such as clinical chemistry, cytogenetics, immunology, molecular genetics, endocrinology, obstetrics, neonatology, neurology, pediatrics, oncology, hematology, anatomic pathology, and infectious diseases. The company works on a 24×7 model and processes between 30,000 & 35,000 blood and fluid samples a day.
-
Sonic Healthcare - Sonic Healthcare provides radiology & laboratory pathology services and follows a federation model with largely decentralized operations. The company is divided into a decentralized federation of medically led practices. It is the third-largest pathology/laboratory medicine company with operations in eight countries. The company employs around 800 specialist pathologists, over 200 radiologists, and others as general practitioners & medical scientists with technicians. In 2007, the company acquired Bioscientia Institut für Medizinische Diagnostik GmbH.
-
Charles River Laboratories - Charles River Laboratories International offers a wide range of clinical and preclinical laboratory services to biotechnology industries, government agencies, and pharmaceutical & medical devices companies. It was incorporated in 1947 and is headquartered in Massachusetts, U.S. The company’s key business areas are basic research, clinical support, and process manufacturing. Its business is segmented into research models & services, discovery & safety assessment, and manufacturing support. Over 60% of revenue is generated from the discovery & safety assessment segment. The company has 65 facilities across 16 countries. It employs over 17,000 people globally.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Clinical Laboratory Service Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2022, historic information from 2018 to 2021, and forecast from 2023 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Clinical Laboratory Service Market Report Assumptions:
-
The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





