- Home
- »
- Clinical Diagnostics
- »
-
Pancreatic Cancer Diagnostic Market Size Report, 2030GVR Report cover
![Pancreatic Cancer Diagnostic Market Size, Share & Trends Report]()
Pancreatic Cancer Diagnostic Market Size, Share & Trends Analysis Report By Product, By Test Type (Imaging Test, Biopsy, Blood Test, Others), By Cancer Type (Exocrine, Endocrine), By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-079-1
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2021
- Forecast Period: 2023 - 2030
- Industry: Healthcare
Pancreatic Cancer Diagnostic Market Trends
The global pancreatic cancer diagnostic market size was valued at USD 2.37 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.53% from 2023 to 2030. The increasing prevalence of pancreatic cancer and the rise in awareness regarding early disease diagnosis is the leading factor driving the global market. In addition, technological advancements in terms of accuracy & sensitivity of diagnostic tests, evolution in molecular diagnostics, and biomarker tests used for malignancy detection are other factors propelling the market demand forward.
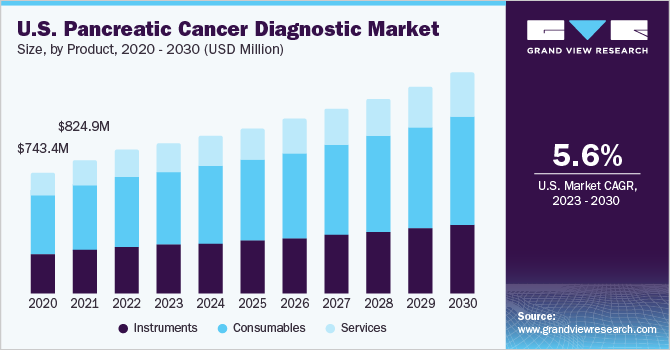
Pancreatic cancer is one of the leading causes of cancer-related deaths globally and the burden of disease is escalating at a significant pace. According to the American Cancer Society, the 5-year relative survival rate of patients having localized pancreatic cancer is around 44%, whereas, in the case of regional and distant stages, the 5-year relative survival rate is 15% and 3% respectively. Therefore, medical professionals and healthcare authorities are emphasizing developing & commercializing precise screening methods to monitor the prevalence levels.
Moreover, early disease diagnosis is very crucial for positive treatment outcomes. As a result, market players and healthcare organizations are raising funds, awareness programs, and research initiatives to promote routine checkups and screenings. For instance, in August 2022, the Pancreatic Cancer Action Network (PanCAN) awarded USD 10.5 million for 16 new grants that would support R&D activities for early detection and better treatment options in cases of pancreatic cancer.
The significant rise in the number of pancreatic malignancy cases is fueling the adoption of screening tests and creating a demand for novel screening tests. According to the National Cancer Institute, in 2022, the number of estimated new cases was 62,210, with 49,830 deaths in the U.S. Currently, diagnostic tests like biomarker tests, liver function tests, biopsies, imaging tests, and others are used in disease diagnosis.
Moreover, the increasing demand for advanced diagnostic solutions has compelled manufacturers to develop novel & precise testing solutions. For instance, in January 2023, Biological Dynamics announced that its lab-on-a-chip plasma test developed for the early-stage detection of pancreatic cancer had entered human trials. This test can detect malignancy at an early stage with accurate biomarkers detection.
Moreover, the increasing number of programs to create awareness about malignant tumor screening is facilitating the demand for diagnostic products worldwide. Partnerships and collaborations undertaken by government & non-government bodies such as WHO, CDC, and Pancreatic Cancer Action Network to promote pancreatic cancer diagnosis are anticipated to fuel market growth.
In addition, various countries have undertaken certain measures to promote the early screening of malignancies. For instance, in October 2018, the UK government announced several measures to diagnose oncology diseases at their early stages, and such initiatives are anticipated to increase the demand for pancreatic cancer testing products.
Advancements in molecular diagnostics are also creating opportunities in the market. Molecular diagnostic tests can detect specific genetic mutations and biomarkers associated with pancreatic cancer, enabling earlier diagnosis and more precise treatment. For example, liquid biopsy tests that detect circulating tumor DNA are being developed that could help diagnose pancreatic cancer at an earlier stage.
Moreover, the use of AI in imaging, pathology tests, and biomarkers tests for diagnosing pancreatic malignancy will boost the adoption of diagnostic tests at the early stages of the disease. According to a study about Artificial Intelligence in Gastroenterology published in April 2021, AI applications in endoscopic ultrasound and novel biomarkers tests to detect pancreatic lesions early showed impressive results, when compared to traditional methods.
Product Insights
The consumables segment held the largest revenue share of 48.4% in 2022 and is anticipated to grow at a steady rate during the projected period. Rising adoption of consumables in diagnostic procedures, an increase in R&D investments, and intensifying launches of technologically advanced diagnostic kits and reagents are factors anticipated to support segment expansion. For instance, in August 2021, Immunovia received approval to start patient testing for the ‘IMMray PanCan-d’ blood test used for the early detection of pancreatic cancer.
The services segment is expected to register a lucrative growth rate during the forecast period. The rising focus on research to develop advanced diagnostic methods, incorporation of modules such as AI, use of computer-aided detection tools in imaging modalities for precise detection, and ongoing efforts from organizations to boost survival rates of pancreatic cancer patients are factors fueling segment uptake. For instance, in September 2021, Lee Health and GenesisCare collaborated to improve the survival rates of pancreas malignancy patients, with the launch of a center of excellence.
Test Type Insights
The imaging test segment dominated the global market with a revenue share of 57.4% in 2022. The segment growth is attributed to its ability to allow healthcare professionals to detect and diagnose pancreatic cancer earlier and help to stage cancer, which can improve treatment outcomes and patient survival rates. Several imaging techniques are currently used in pancreatic cancer diagnosis, including computed tomography (CT) scans, magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), and positron emission tomography (PET) scans.
Moreover, these techniques are much preferred by health professionals owing to their higher accuracy in malignancy detection and their non-invasive nature. Advancements in imaging technologies, such as the development of contrast agents and 3D imaging, and the use of AI in imaging, are further improving the accuracy & efficiency of pancreatic cancer diagnosis. Rising efforts from researchers to develop advanced imaging modalities for precise diagnosis is another factor supporting segment growth. For instance, a research team from Taiwan has been studying a CAD tool that uses artificial intelligence to detect pancreatic cancer.
The market for blood tests is projected to experience robust growth during the forecast period. The segment growth is owing to the rising demand for early diagnosis of malignancies and technological advancements. Pancreatic cancer can elevate the level of certain biomarkers, which act as tumor markers in disease diagnosis. For instance, CA 19-9 and carcinoembryonic antigen are the most common type of biomarkers for diagnosing cancers of the pancreas. Moreover, the rising emphasis on genetic tests and counseling has resulted in the deeper market penetration of blood tests such as Galleri and PanCan-d.
Cancer Type Insights
The exocrine cancer segment dominated the market with a revenue share of 93.5% in 2022. Factors such as the high prevalence of exocrine malignancies, the development of new & more effective diagnostic technologies, and a growing awareness regarding the importance of early detection and treatment of pancreatic cancers are factors responsible for the higher market share of the exocrine segment.
According to Johns Hopkins University, all types of exocrine cancer account for more than 95% of cancers of the pancreas. Out of these, adenocarcinoma is the most common form, accounting for 90% of the diagnostic cases. Thus, the higher incidence of adenocarcinoma propels the disease diagnosis rate, thereby driving segment growth.
On the other hand, the endocrine segment is expanding at a steady rate. Endocrine malignancies are not as common as exocrine ones and account for less than 5% of all pancreas tumors. However, significant lifestyle changes, inherited gene mutations, and the presence of comorbidities are expected to increase the disease prevalence and drive segment growth in the coming years.
End-use Insights
The hospitals segment dominated the pancreatic cancer diagnostics market with a revenue share of 56.5% in 2022. Factors such as rising awareness regarding personalized medicines, improvements in affordable healthcare services, an increasing number of patient visits at hospitals, and a growing focus on strategic activities are supporting the hospital segment growth.
For instance, in December 2021, the City of Hope cancer center announced that it had agreed to acquire Cancer Treatment Centers of America to build a national, integrated research and treatment system for cancer. The joint entity will be one of the major cancer treatment and research organizations in the U.S. with a strong geographic footprint, serving nearly 115,000 patients each year.
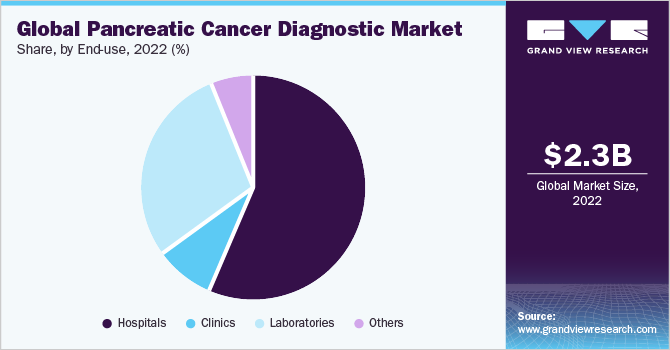
The laboratories segment is likely to be the fastest-growing segment during the forecast period. The high disease burden is fueling the demand for lab tests for accurate disease diagnosis. In addition, the large volume of tests performed in labs, advanced healthcare infrastructure acquired by laboratories, and favorable reimbursement policies are supporting the segment expansion. For instance, according to the American Society of Clinical Oncology, the cost for most of the genetic testings are covered by health insurance plans.
Regional Insights
North America held a market share of 40.41% in 2022. The growth of the North American region is fueled by the increasing incidence of the target disease and rising initiatives from profit and non-profit organizations to promote early disease diagnosis. For instance, the Pancreatic Cancer Action Network is a leading organization that promotes research activities and creates awareness about the condition.
Moreover, the presence of leading market players and various strategic initiatives undertaken by them is another factor supporting the regional market growth. For instance, Abbott, Agilent Technologies, Inc, and Laboratory Corporation of America Holdings have a well-established position in the regional market. Furthermore, the increasing demand for novel diagnostic tests and favorable reimbursement policies are also expected to support market growth in North America.
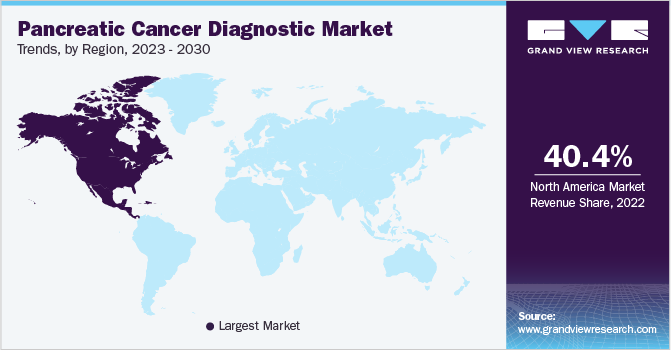
The heavy burden of pancreatic cancer, high unmet needs, and increasing investments by market players in the Asia Pacific region are factors driving the APAC market. For instance, in December 2022, Hirotsu Bio Science, a biotechnology company in Japan, developed the first early screening tests for pancreas malignancies. Additionally, the increasing demand for minimally invasive tests and supportive government legislations are further escalating the regional market advancement.
Key Companies & Market Share Insights
Notable industry players are adopting strategies such as new product development, mergers & acquisitions, and partnership strategies to increase their market share. Market players such as Illumina, Inc., Agilent Technologies, Inc., F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific, Inc., and QIAGEN dominated the global market. These key players have been developing advanced testing solutions to meet the evolving global demand for novel diagnostic solutions.
They have been introducing various innovations in the market and expanding their product portfolios to stay competitive. For instance, in January 2023, Mayo Clinic announced the assessment of the radiotracer, 68Ga-Fibroblast-Activation-Protein-Inhibitors (FAPI)-46, in pancreatic cancer imaging in a clinical trial. Some prominent players in the global pancreatic cancer diagnostic market include:
-
Thermo Fisher Scientific, Inc.
-
QIAGEN
-
Illumina, Inc.
-
F. Hoffmann-La Roche Ltd.
-
BD
-
Agilent Technologies, Inc.
-
Myriad Genetics, Inc
-
Koninklijke Philips N.V.
-
Abbott
-
Hitachi, Ltd.
-
Danaher
-
Prestige Biopharma Ltd.
-
BioMarker Strategies
-
ASURAGEN, INC
Pancreatic Cancer Diagnostic Market Report Scope
Report Attribute
Details
Market size value in 2023
USD 2.50 billion
Revenue forecast in 2030
USD 3.90 billion
Growth rate
CAGR of 6.53% from 2023 to 2030
Base year for estimation
2022
Historical data
2018 - 2021
Forecast period
2023 - 2030
Quantitative units
Revenue in USD billion, and CAGR from 2023 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, test type, cancer type, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; India; China; Japan; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; UAE; South Africa; Kuwait
Key companies profiled
Thermo Fisher Scientific, Inc.; QIAGEN; Illumina, Inc.; F. Hoffmann-La Roche Ltd.; Agilent Technologies, Inc.; Abbott; BD; Myriad Genetics, Inc; Koninklijke Philips N.V.; Hitachi, Ltd; Danaher; Prestige Biopharma Ltd; BioMarker Strategies; ASURAGEN, INC
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Pancreatic Cancer Diagnostic Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global pancreatic cancer diagnostic market report based on product, test type, cancer type, end-use, and region:
-
Product Outlook (Revenue, USD Billion, 2018 - 2030)
-
Instruments
-
Consumables
-
Services
-
-
Test Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Imaging Test
-
CT Scan
-
MRI
-
Ultrasound
-
PET
-
Others
-
-
Biopsy
-
Blood Test
-
Liver Function Tests
-
Tumor Markers
-
Others
-
-
Others
-
-
Cancer Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Exocrine
-
Adenocarcinoma
-
Squamous Cell Carcinoma
-
Adenosquamous Carcinoma
-
Colloid Carcinoma
-
-
Endocrine
-
-
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospitals
-
Clinics
-
Laboratories
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
U.K.
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The pancreatic cancer diagnostic market size was estimated at USD 2.37 billion in 2022 and is expected to reach USD 2.50 billion in 2023.
b. The pancreatic cancer diagnostic market is expected to grow at a compound annual growth rate of 6.53% from 2023 to 2030 and is expected to reach USD 3.90 billion by 2030.
b. The consumables segment is expected to dominate the pancreatic cancer diagnostic market with a share of 48.40% in 2022 due to the increasing adoption of novel tests, increased R&D investments, and rising launch of technologically advanced diagnostic kits and reagents.
b. Some key players operating in the pancreatic cancer diagnostic market include Thermo Fisher Scientific, Inc., QIAGEN, Illumina, Inc., F. Hoffmann-La Roche Ltd., Agilent Technologies, Inc., BD, Myriad Genetics, Inc, Koninklijke Philips N.V., and Abbott among others.
b. Increasing prevalence of pancreatic cancer, rise in awareness for early disease diagnosis, and advancements in molecular diagnostics are the major factors driving the pancreatic cancer diagnostic market growth over the forecast period.
b. North America held the largest share of 40.41% in 2022 and is expected to register a lucrative growth rate over the forecast period. It is attributable to the increasing incidence of disease, presence of leading market players, and rising initiatives from profit and non-profit organizations to promote early disease diagnosis.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





