- Home
- »
- Healthcare IT
- »
-
Pharmacovigilance Market Size, Share & Trends Report 2030GVR Report cover
![Pharmacovigilance Market Size, Share & Trends Report]()
Pharmacovigilance Market Size, Share & Trends Analysis Report By Product Life Cycle, By Service Provider (In-house, Contract Outsourcing), By Type, By Therapeutic Area, By Process Flow, By End Use, By Region, And Segment Forecasts, 2024 - 2030
- Report ID: 978-1-68038-327-0
- Number of Report Pages: 298
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Pharmacovigilance Market Size & Trends
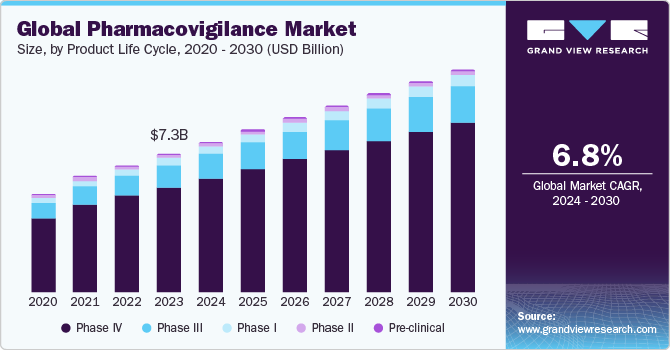
The global pharmacovigilance market size was estimated at USD 7.32 billion in 2023 and is anticipated to grow at a CAGR of 6.8% from 2024 to 2030. The rising incidence of Adverse Drug Reactions (ADRs) owing to drug abuse and the prevalence of diseases that require a combination of drugs are the major growth drivers for the market. In addition, an upward shift in the production of novel drugs and the presence of stringent government regulatory frameworks for drug safety are significantly boosting the market growth. For instance, the U.S. FDA and the EU’s European Medical Agency (EMA) formulate regulatory guidelines for all phases of clinical trials. Moreover, advancements in the development of ADR databases and information systems have enabled accurate reporting of information, which can be further utilized by research professionals for prospective clinical studies, thereby fueling overall growth.
A rise in the incidence of chronic diseases, such as cancers, diabetes, and cardiovascular & respiratory disorders, has led to an increase in drug consumption worldwide. According to a WHO report on pharmaceutical consumption, medicines to treat chronic diseases accounted for a larger proportion of the total volume of drug consumption in nonhospital setups. Increasing drug development activities in areas such as personalized medicines, biosimilars, orphan drugs, and companion diagnostics, along with adaptive trial designs, is projected to boost the demand for pharmacovigilance services in the coming years.
Furthermore, the increasing incidence of ADR and drug toxicity is fueling the market growth. According to the National Center for Biotechnology Information (NCBI), approximately 5% of total hospitalizations in a year are due to ADR in Europe. Furthermore, a February 2022 article published in the Journal of Current Medicine Research and Practice titled "Characterization of Seriousness and Outcome of Adverse Drug Reactions in Patients Receiving Cancer Chemotherapy Drugs - A Prospective Observational Study" revealed that serious Adverse Drug Reactions (ADRs) in the U.S. result in over 100,000 deaths annually and have been a major health concern since the past decade.
Adverse Drug Events (ADEs) in hospitals
Hospital Settings
Inpatient
Outpatient
Statistics
ADEs accounted for an estimated:
- 1 in 3 of all hospital adverse events
- Affect about 2 million hospital stays each year
- Prolong hospital stays by 1.7 to 4.6 days
ADEs in outpatient settings account for:
- Over 3.5 million physician office visits
- An estimated 1 million emergency department visits
- Approximately 125,000 hospital admissions
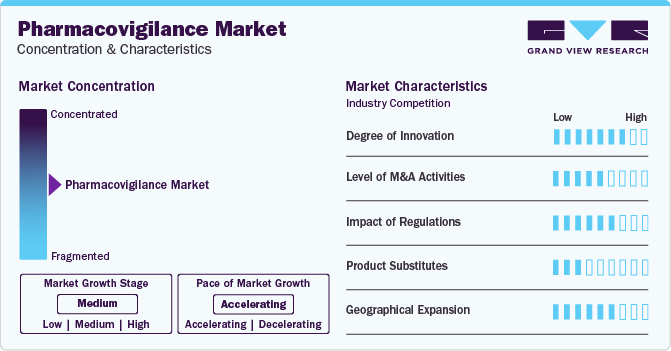
Market Concentration & Characteristics
The pharmacovigilance market is currently experiencing moderate growth at an accelerating pace. This growth is attributed to factors such as rising drug consumption and development rates, a higher incidence of adverse drug reactions (ADR) and drug toxicity, and an increasing trend in outsourcing pharmacovigilance services. Outsourced services include medical writing, clinical trial data collection, medical reporting, and other pharmacovigilance-related services. Manufacturers are actively seeking ways to reduce costs and minimize operational expenses by transitioning from fully integrated pharmaceutical companies to collaborative partnerships with service providers.
Market players are utilizing key strategies, including new product launches, expansions, acquisitions, partnerships, etc. For instance, In January 2023, IQVIA announced a collaboration with Alibaba Cloud, an intelligence and digital technology arm of Alibaba Group. The goal of this collaboration is to deliver commercial clinical solutions in the Chinese market.
Similarly, in February 2022, in order to jointly supply clinical research solutions based on Medable's software-as-a-service platform for decentralized clinical trials, Cognizant and Medable Inc. engaged in cooperation.
“We’re thrilled to partner with Cognizant to empower pharma and biotech teams and accelerate the shift to patient-centric, decentralized clinical trials. Cognizant’s strong life sciences expertise, global implementation resources, and change management capabilities will help ensure that clinical trial sponsors receive best-in-class capabilities to maximize their success.”
- Dr. MaryAnne Rizk, chief strategy officer, Medable
The pharmacovigilance landscape is witnessing increased innovation, incorporating big data analytics, cloud-based solutions, artificial intelligence, automation, and digitalized medicines. The integration of analytical techniques and big data is poised to propel traditional pharmacovigilance practices forward. Future developments include the creation of algorithms for signal detection from various sources, with AI technologies revolutionizing PV science through intelligent signal evaluation.
Mergers and acquisitions are increasing in the pharmacovigilance market. Companies are strategically acquiring others to bolster product offerings, expand global reach, diversify portfolios, integrate technologies, and enhance their standing in the industry. For instance, in April 2023, a merger between Pharmalex GmbH and Cpharm Australia, allowed Pharmalex Group to extend its presence in Australia and New Zealand, catering to organizations of varying sizes. This move enhances coverage of pharmacovigilance and medical devices in the Oceania region.
Pharmacovigilance outsourcing services insights
Small
Medsize
Enterprise
Inhouse PV
Limited
Small Team
Large Global Organization
Primary Outsourcing Goal
PV expertise and safety database
Scale
Cost reduction
Key Capabilities of modern safety solutions
Built in industry processes and best practices
Seamlessly scales with more cases, users, content, etc.
Easier and faster to upgrade and validate new releases
Direct data access for internal and external users
Easy to configure and modify business process
AI and automation to reduce manual work
The pharmacovigilance market operates within a highly regulated framework. In the U.S., regulatory oversight is managed by the U.S. Department of Health and Human Services and the FDA. The U.S. FDA, in conjunction with the Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER), monitors pharmacovigilance activities. The escalating demand for pharmacovigilance solutions for adverse drug reaction (ADR) reporting is anticipated to intensify regulatory compliance pressures on healthcare providers throughout the forecast period.

North America and Europe held the prominent share in the pharmacovigilance market, the Asia-Pacific region is witnessing rapid growth due to expanding pharmaceutical industries and favorable regulatory environments. As regulatory standards evolve globally, regional concentrations in the market may continue to shift toward lucrative growth.
Case Studies
Pharmaceutical companies are forming partnerships to leverage low-code platforms and AI-powered decision-making & workflow automation systems. This strategic move aims to streamline development processes and reduce efforts.

A Case Study on “Pfizer: Managing Drug Safety Across Global Partnerships”
- Initiative
o Automating pharmacovigilance agreement management through the rapid delivery of an integrated solution.
- Implementation
o In less than 4 months, Pfizer developed its Pharmacovigilance Exchange (PVX) solution using Pega's Business Process Management and Dynamic Case Management tools and their pharmacovigilance application.
o Pfizer could utilize existing components from previous Pega systems due to Pega's Direct Capture of Objectives and Situational Layer Cake approach, which reduced development efforts by about 30%.
- Outcome
o By automating several procedures, the PVX program has greatly decreased manual effort for Pfizer. It manages the preparation, evaluation, approval, and update of Pharmacovigilance Agreements (PVAs). Important deadlines and milestones are automatically tracked, boosting compliance & transparency.
o The software also assigns roles & deadlines and records & controls pharmacovigilance operations, including Aggregate Reports, Risk Management, and Label Updates.
o Pega's system interfaces with Pfizer's existing PV systems for data access and sends notifications for impending events. The platform provides thorough reporting & audit features, increasing transparency and minimizing audit efforts.
Product Life Cycle Insights
Phase IV (post-marketing) segment dominated the overall pharmacovigilance market in the product life cycle segment with over 75.9% revenue share in 2023. The phase IV trial is crucial in the entire clinical trial process as unsuspected ADRs can be detected in this stage. This can be attributed to intensive drug testing on a large patient demographic of the highest relevance post the drug commercialization. A phase IV study is conducted on a nonmedicated population. Some examples of phase clinical studies are:
-
Investigation of subsets of drugs indicated for approved patient groups.
-
Market research studies of competitor drugs
-
Demographically specific studies in comparison to another drug or treatment
-
Investigation of a specific AE that has occurred after commercialization
Phase III segment in the product life cycle segment is projected to witness growth at a lucrative CAGR during the forecast period. Phase III trials are conducted to specify and establish drug efficacy. These trials provide supplementary information about drug safety, possible drug interactions, and pre-commercialization effectiveness. In addition, players operating in the segment are undertaking strategic initiatives to integrate effective trial management practices in drug development and trials. For instance, in June 2022, Florence Healthcare raised USD 27 million to support the expansion of its technology platform to integrate the growing demand for clinical trials. These factors are anticipated to drive the segment over the forecast period.
Service Provider Insights
The contract outsourcing segment held the largest market share of 60.4% in 2023. The segment dominated the market owing to the rapid entry of multiple Contract Research Organizations (CROs), which provide end-to-end clinical trial solutions, especially in key Asia Pacific economies of India, China, & Japan. The segment is anticipated to grow at the fastest CAGR over the forecast period, as contract outsourcing partners offer a balanced and flexible solution within cost-contained models, ensuring overall quality. Pharmacovigilance outsourcing now encompasses intricate tasks, including benefit-risk management, signal detection, pharmacoeconomics, and comprehensive risk management planning.
Type Insights
The spontaneous reporting segment dominated the market in 2023 with a share of 30.2%, owing to its wide-scale usage in detecting new, serious, and rare ADRs efficiently & affordably. The growing use of surveillance reports developed through this procedure by regulatory authorities and pharmaceutical industries is responsible for the significant market share of spontaneous reporting.
The targeted spontaneous reporting segment is anticipated to witness a lucrative CAGR during the forecast period, owing to the rising government initiatives to incorporate reporting methodologies other than spontaneous reporting by the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP). Associated benefits such as greater affordability, lower labor costs, feasibility in poor resource settings, and usage in routine monitoring are expected to drive the demand over the forecast period. These factors are contributing to a steady growth of this segment.
Therapeutic Area Insights
The oncology segment dominated the pharmacovigilance market, with a share of 26.9% in 2023. It is also expected to be the fastest-growing segment over the forecast period. Pharmacovigilance in oncology refers to the specialized monitoring and assessment of safety data related to anticancer drugs and therapies. It involves the systematic collection and analysis of adverse events specific to oncology patients, identification of potential drug-related risks, and proactive risk management strategies to ensure cancer treatments' safe and effective use.
An increasing number of research activities undertaken by various biopharmaceutical firms for cancer treatments and the rising government support to improve the living conditions of people living with cancer propelling the research activities is anticipated to accelerate the need for pharmacovigilance in clinical research activities. For instance, the White House in 2023 announced an ambitious project to reduce the death rate of Cancer by at least 50.0% in the next 25 years. In addition, USD 2 billion was granted for R&D purposes in the Cancer Moonshot initiative, which will support clinical, laboratory, public health, and others for cancer treatment. These factors are responsible for the growth and dominance of the segment during the forecast period.
Process Flow Insights
The signal detection segment held the largest revenue share in 2023. Signal detection refers to identifying various safety signals and actively searching for such signals across registries and plausible healthcare data sources. Regulators require biopharmaceutical firms to maintain spontaneous reporting systems, and most commonly, signals are generated from such systems. Furthermore, data for processes can be stored in databases maintained by the pharmaceutical firm, a technology partner, or a contract research organization. The signal source may vary from spontaneous reporting, interventional studies, and clinical & non-clinical studies to literature, social media, free text, etc.; thus, effective signal detection is important.
The case data management segment is anticipated to grow at a lucrative CAGR in the coming years. As adverse event information can be produced from diverse modes such as spontaneous reports, clinical trials, post-marketing programs, and literature. In addition, emerging technologies such as artificial intelligence (AI) and machine learning are widely used for case data management. Some data management software include repClinical, PvNET, Siebel Clinical, ClinSource, Oracle, and more.
End Use Insights
The pharmaceuticals segment dominated the market with a revenue share of over 44.0% in 2023. Pharmacovigilance for pharmaceutical companies involves systematically collecting, monitoring, and evaluating safety data related to their marketed drugs or investigational products. It ensures compliance with regulatory requirements, promotes patient safety, and supports the ongoing evaluation and optimization of drug safety profiles. Moreover, pharmacovigilance allows pharmaceutical firms to limit drug development costs, as it provides an early warning system.
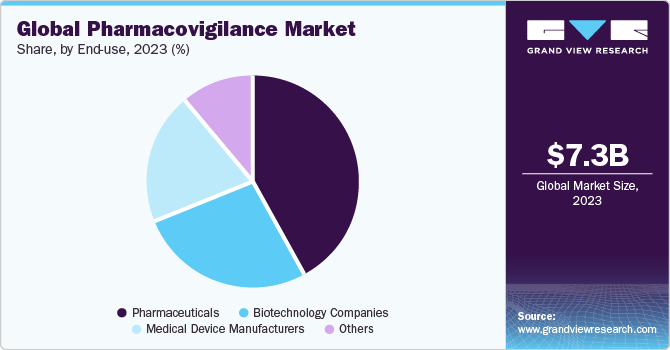
The biotechnology companies segment is expected to grow at the fastest CAGR during the forecast period. Biotechnology firms prioritize the development of innovative products, such as novel therapies, biologics, and gene therapies. These products often leverage cutting-edge technologies and may target rare diseases or feature unique mechanisms of action. For instance, in December 2022, the U.S. FDA approved ADSTILADRIN biologic manufactured by Ferring Pharmaceuticals A/S. This biologic is indicated for use in adult patients with high-risk BCG-unresponsive nonmuscle invasive bladder cancer. Such advancements are expected to propel the segment growth over the forecast period.
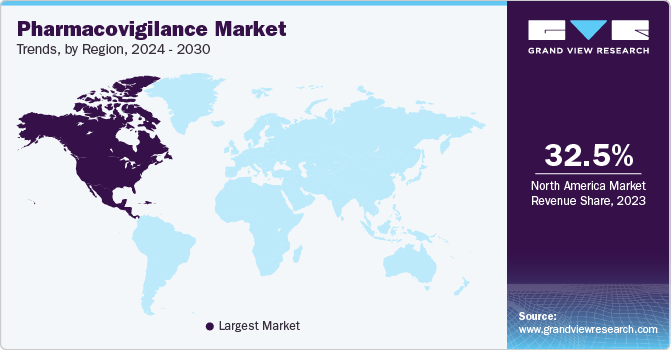
Regional Insight
North America dominated the pharmacovigilance market in 2023 and held the largest market share of over 32.5%. This can be attributed to the presence of key pharmaceutical players in this region, which results in a major contribution to the overall revenue generated by this region. The rise in drug abuse and associated ADRs is a leading cause of morbidity and mortality. The abovementioned elements act as high-growth rendering factors for North America's Pharmacovigilance (PV) market. Furthermore, growing patient awareness and concerns related to the safety of drugs are expected to positively impact the market.
U.S. Pharmacovigilance Market Trends
Pharmacovigilance market in the U.S. held the largest market share in 2023, owing to high drug consumption and strict regulatory environment. The U.S. FDA strictly regulates pharmacovigilance activities for drugs and medical devices for New Drug Applications (NDA). Implementation of acts and regulations such as Risk Evaluation and Mitigation Strategy (REMS) Act to unveil the potential risks associated with a drug following a series of drug safety-related events. Drugs that fail to follow the REMS provision could be considered misbranded. Such factors are expected to fuel the market growth.
Europe Pharmacovigilance Market Trends
The pharmacovigilance market in Europe is driven by stringent regulatory requirements, increasing drug development, and growing awareness of drug safety. In 2020, 16,256 clinical trials were registered, primarily by the European pharmaceutical industry. Moreover, in 2021, 3.5 million adverse ADRs were received by EudraVigilance, a 93% increase from the previous year. The European Medicine Agency (EMA) has implemented a Risk Management Plan (RMP) for the PV system, requiring approval for every drug or biologic before commercialization. The EMA has also launched www.adrreports.eu, a new public website that publishes suspected adverse effects of all medicines and active substances authorized under the European Economic Area. Furthermore, the growing demand for clinical trials and the need for novel drug development due to the geriatric population and an increasing number of pharmaceutical and biotechnology firms are the factors driving the market growth in the region.
The UK pharmacovigilance market is expected to grow over the forecast period, due to the well-established healthcare system and increasing incidence of medication errors and ADRs. The increasing number of clinical trials and the Yellow Card Scheme by the UK government also contribute to market growth. The Medicines and Healthcare Products Regulatory Agency (MHRA) inspects MAHs and verifies compliance with UK PV regulations, conducting Good Pharmacovigilance Practice (GPvP) inspections.
The pharmacovigilance market in Germany held the largest revenue share in 2023. The presence of government bodies, such as Deutsche Forschungsgemeinschaft (DFG), which is a German research funding organization that offers funds for clinical trials, is expected to further propel the market growth. The Federal Institute for Drug and Medical Devices (BfArM) has also launched a database UAW-DB, which publishes the suspected adverse effects of all drugs and active substances along with suspected cases of ADRs in Germany since 1995. This initiative is in line with BfArM’s plan to streamline the regulatory process in Germany. Such initiatives are expected to propel the industry growth in the coming years.
Asia Pacific Pharmacovigilance Market Trends
Asia Pacific is expected to witness lucrative growth in the forecast period. Asia provides a substantial cost-saving advantage, with savings ranging from 50% to 80% of the cost compared to developed nations, thus leading to an increase in the number of clinical trials being conducted in this region. Hence, the rise in demand for clinical trials has led to an increasing focus on PV and drug safety in the region. Along with India and China, Singapore, South Korea, and Taiwan are recognized as outsourcing hubs in Asia Pacific.
The pharmacovigilance market in China is the largest in the Asia Pacific region due to the rising incidence of ADRs, rising healthcare expenditure, and government initiatives. According to the National Medical Products Administration (NMPA) April 2020 article, in 2019, around 97% of country-level regions reported ADRs. Nonprofit organizations like the Chinese Clinical Trial Registry (ChiCTR) contribute to market growth. The biotechnology sector's growth and government incentives for local pharmaceutical companies to adopt PV practices further drive the market. Economic development and growing disposable income make China the fastest-growing market in the region. The National Medical Products Administration (NMPA) issued Good Pharmacovigilance Practices (GVP) in December 2021, mandating compliance for MAHs and drug registration applicants.
The Japan pharmacovigilance market is expected to grow significantly over the forecast period, due to factors such as chronic disease prevalence and high drug consumption. Furthermore, an increase in commercial R&D activities for drug development is expected to boost the market over the forecast period. The Japan Medical Association Centre for Clinical Trials (JMACCT) has undertaken a “Large Scale Clinical Trial Network Project,” which is a clinical trial promotion project aimed at implementing educational projects to increase awareness regarding clinical trials and promote clinical trial networks.
The pharmacovigilance market in India is the fastest-growing market for PV services in Asia Pacific. The rising incidence of adverse drug reactions (ADRs) and heightened awareness among healthcare professionals contribute to market growth. For instance, In June 2024, India's Central Drugs Standard Control Organization (CDSCO) issued a draft guidance document for the industry on pharmacovigilance requirements for human vaccines. This guidance outlines the responsibilities of all stakeholders, including Marketing Authorization Holders, in vaccine safety monitoring, developing risk management plans, conducting audits and inspections, and periodically submitting a Risk Benefit Evaluation Report (PSUR) to the Licensing Authority. It highlights the necessity of continuous vigilance over vaccine products to ensure their safety and efficacy.
Key Pharmacovigilance Company Insights
The market is characterized by a few notable players, including Accenture, IQVIA, Cognizant, Aris Global, and IBM Corporation. These manufacturers are actively utilizing strategic initiatives such as mergers and acquisitions to strengthen their market positions. For instance, in October 2023, IQVIA strategically collaborated with argenx to advance treatment to patients with rare autoimmune diseases through innovative and integrated technology-enabled pharmacovigilance (PV) safety services and solutions.
"We look forward to collaborating closely with IQVIA on this important business need. We aim to innovate in all that we do and IQVIA’s technology-enabled PV services and solutions will allow for efficient data integration as we work to bring new treatment options to autoimmune patients”.
- Tim Van Hauwermeiren, CEO, argenx.
In November 2022, Linical Americas (a U.S. subsidiary of The Linical Group) and Science 37 Holdings, Inc. announced a partnership to enable the deployment of hybrid and fully decentralized trials. This partnership will provide enhanced access to Linical’s offerings.
“By partnering with Linical, we have an important new ally in our mission to accelerate clinical research and enable universal access for patients,” “Our technology-enabled Metasite will empower and enhance Linical’s solutions, helping patient’s access new life-changing treatments quicker, in the largest and most prevalent therapeutic areas.”
- ”David Coman, Chief Executive Officer of Science 37
Recent Developments
- In March 2023, ICON plc and LEO Pharma announced partnerships to impel execution of clinical trials in medical dermatology space.
“We’ve been exploring several outsourcing models but found a hybrid sourcing model to be the most efficient. Partnering with ICON supports our 2030 strategy as it will help us to bring innovative treatments to patients faster while also supporting a more sustainable business through scalability and flexibility. “ICON’s wealth of services and leading position in clinical development will support LEO Pharma’s R&D strategy building on driving innovation through partnerships and support staying competitive.”
- Jörg Möller, Executive Vice President and head of Global R&D at LEO Pharma
- In February 2023, Parexel International Corporation announced the launch of Expert Series-New Medicines, Novel Insights. The series features latest insights from company’s cross-functional experts postanalysis of trends that impact drug development and evidence-based guidance for the biopharmaceutical industry.
“Cutting-edge medicines are becoming more personalized and precise across the therapeutic landscape, while the process to develop those therapies is reaching new heights of complexity. “Parexel’s New Medicines, Novel Insights research series offers expert-led guidance to deliver on the promise of patient-focused drug development and bring impactful treatments to patients more rapidly.”
- Amy McKee, MD, Chief Medical Officer and Head of Oncology Center of Excellence
Key Pharmacovigilance Companies:
The following are the leading companies in the pharmacovigilance market These companies collectively hold the largest market share and dictate industry trends.
- Accenture
- IQVIA Inc.
- Cognizant
- Clinquest Group B.V. (Linical Americas)
- IBM
- Laboratory Corporation of America Holdings
- ArisGlobal
- Capgemini
- ITClinical
- ICON plc.
- TAKE Solutions Limited
- Parexel International (MA) Corporation
- Wipro
- United BioSource LLC
- BioClinica Inc. (Clario)
- ClinChoice (formerly FMD K&L)
Pharmacovigilance Market Report Scope
Report Attribute
Details
The market size value in 2024
USD 7.95 billion
Revenue forecast in 2030
USD 11.78 billion
Growth rate
CAGR of 6.8% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Report updated
June 2024
Quantitative units
Revenue in USD million/billion & CAGR from 2023 to 2030
Report coverage
Revenue forecast, company share, competitive landscape, growth factors, and trends
Segments covered
Service provider, product life cycle, type, process flow, therapeutic area, end use, region
Regional Scope
North America; Europe; Asia Pacific; Latin America; MEA
Country Scope
U.S.; Canada; UK; Germany; France; Italy; Spain; Russia; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina, South Africa; Saudi Arabia; UAE; Kuwait.
Key companies profiled
Accenture; IQVIA; Cognizant; Clinquest Group B.V. (Linical Americas); IBM; Laboratory Corporation of America Holdings; ArisGlobal; Capgemini; ITClinical; ICON plc.; TAKE Solutions Ltd.; PAREXEL International Corporation.; Wipro; United BioSource LLC; BioClinica Inc (Clario).; ClinChoice (formerly FMD K&L)
Customization scope
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Pharmacovigilance Market Report Segmentation
This report forecasts revenue growth at global, regional, & country levels and provides an analysis of the industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global pharmacovigilance market report based on service provider, product life cycle, type, process flow, therapeutic area, end use, and region:
-
Product Life Cycle Outlook (Revenue, USD Million, 2018 - 2030)
-
Pre-clinical
-
Phase I
-
Phase II
-
Phase III
-
Phase IV
-
-
Service Provider Outlook (Revenue, USD Million, 2018 - 2030)
-
In-house
-
Contract Outsourcing
-
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Spontaneous Reporting
-
Intensified ADR Reporting
-
Targeted Spontaneous Reporting
-
Cohort Event Monitoring
-
EHR Mining
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharmaceuticals
-
Biotechnology Companies
-
Medical Device Manufacturers
-
Others
-
-
Therapeutic Area Outlook (Revenue, USD Million, 2018 - 2030)
-
Oncology
-
Neurology
-
Cardiology
-
Respiratory Systems
-
Others
-
-
Process Flow Outlook (Revenue, USD Million, 2018 - 2030)
-
Case Data Management
-
Case Logging
-
Case Data Analysis
-
Medical Reviewing & Reporting
-
-
Signal Detection
-
Adverse Event Logging
-
Adverse Event Analysis
-
Adverse Event Review & Reporting
-
-
Risk Management System
-
Risk Evaluation System
-
Risk Mitigation System
-
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Russia
-
Rest of Europe
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
Rest of Asia Pacific
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
Rest of Latin America
-
-
The Middle East and Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
Rest of MEA
-
-
Frequently Asked Questions About This Report
b. The global pharmacovigilance market size was estimated at USD 7.32 billion in 2023 and is expected to reach USD 7.95 billion in 2024.
b. The global pharmacovigilance market is expected to grow at a compound annual growth rate of 6.8% from 2024 to 2030 to reach USD 11.78 billion by 2030.
b. North America dominated the pharmacovigilance market with a share of over 32.00% in 2023. This is attributable to rising drug abuse and related Adverse Drug Reactions (ADRs), increasing patient awareness and safety concerns, and rising strategic initiatives by key market players in the region.
b. Some key players operating in the pharmacovigilance market include Accenture; IQVIA; Cognizant; Clinquest Group B.V. (Linical Americas); IBM; Laboratory Corporation of America Holdings; ArisGlobal; Capgemini; ITClinical; ICON plc.; TAKE Solutions Ltd.; PAREXEL International Corporation; Wipro; United BioSource LLC; BioClinica Inc.(Clario); ClinChoice (formerly FMD K&L).
b. Key factors that are driving the pharmacovigilance market growth include growing drug consumption and drug development rates, increasing incidence of ADR and drug toxicity, rising trend of outsourcing pharmacovigilance services, increased externalization of clinical trial studies by large pharmaceutical and biopharmaceutical companies, the growing regulatory burden on manufacturers and introduction of advanced software services, constantly rising investment in R&D by healthcare companies and increasing partnerships and collaborations between market players.
Table of Contents
Chapter 1 Research Methodology & Scope
1.1 Market Segmentation & Scope
1.1.1 Product Life Cycle
1.1.2 Service Provider
1.1.3 Type
1.1.4 Process Flow
1.1.5 Therapeutic Area
1.1.6 End Use
1.1.7 Regional Scope
1.1.8 Estimates and Forecast Timeline
1.2 Research Methodology
1.3 Information Procurement
1.3.1 Purchased Database:
1.3.2 GVR’s Internal Database
1.3.3 Secondary Sources
1.3.4 Primary Research:
1.3.5 Details Of Primary Research
1.3.5.1 Data for primary interviews in North America
1.3.5.2 Data for Primary Interviews in Europe
1.3.5.3 Data for Primary Interviews in Asia Pacific
1.3.5.4 Data for Primary Interviews in Latin America
1.3.5.5 Data for Primary Interviews in Middle East & Africa
1.4 Information or Data Analysis
1.4.1 Data Analysis Models
1.5 Market Formulation & Validation
1.6 Model Details
1.6.1 Commodity Flow Analysis
1.6.1.1 Top down market estimation
1.6.1.2 CAGR Calculation
1.6.1.3 Key Report Updates
1.7 List of Secondary Sources
1.8 List of Abbreviations
1.9 Market Definitions
1.10 Report Objectives
1.10.1 Objective 1:
1.10.2 Objective 2:
Chapter 2 Executive Summary
2.1 Market Snapshot
2.2 Segment Snapshot (Product Life Cycle & Service Provider)
2.3 Segment Snapshot (Type & Therapeutic Area)
2.4 Segment Snapshot (Process Flow & End Use)
2.5 Competitive Landscape Snapshot
Chapter 3 Pharmacovigilance Market Variables, Trends & Scope
3.1 Market Lineage Outlook
3.1.1 Parent Market Analysis
3.1.2 Ancillary Market Analysis
3.2 Penetration & Growth Prospect Mapping
3.3 Pharmacovigilance Market Dynamics
3.3.1 Market Driver Analysis
3.3.1.1 Growing drug consumption and drug development rates
3.3.1.2 Increasing incidence of ADR and drug toxicity
3.3.1.3 Increasing trend of outsourcing pharmacovigilance services
3.3.1.4 Increasing externalization of clinical trial studies by large pharmaceutical and biopharmaceutical companies
3.3.1.5 Increasing regulatory Burden on Manufacturers
3.3.1.6 Introduction of technologically advanced software services
3.3.1.7 Constantly rising investment on R&D by healthcare companies
3.3.1.8 Partnerships and collaborations between market players
3.3.2 Market Restraint Analysis
3.3.2.1 Shortage of skilled labor
3.3.2.2 Expensive technology for small and mid-sized player
3.3.2.3 Lack of recognition
3.3.2.4 Scarcity of integration standards
3.3.3 Industry Challenges
3.4 Pharmacovigilance Market Analysis Tools: Porters
3.5 SWOT Analysis, by Factor (Political & Legal, Economic, and Technological)
3.6 Value Chain Analysis
3.6.1 Preclinical
3.6.2 Clinical
3.6.3 PMA
3.7 Mapping of Life Cycle against Service Offering and Their Demand
3.8 Regulatory Framework
3.8.1 List of Regulatory Bodies by Country
3.9 Organization Structure Introduction
3.10 Pricing Models
3.10.1 Drug Safety Budget Allocation by Activities
3.10.2 By Development Phase
3.10.3 By Therapeutic Area
3.10.4 Pricing Level
3.10.4.1 Project management
3.10.4.2 Case processing
3.10.4.3 ADR Reporting
3.10.4.4 Medical writing
3.10.4.5 Drug safety management
3.11 Technology Timeline Overview
3.11.1 Changing Technology & Adoption
3.11.1.1 Social Media
3.11.1.2 Literature screening
3.11.1.3 Automation and AI
3.11.1.4 Big data analytics in PV
3.12 Impact of COVID-19
3.12.1 Recent Developments & Strategic Outcomes
3.12.1.1 Regulatory requirements/changes due to covid-19
3.12.2 Strategies Implemented by Companies
3.12.2.1 IQVIA
3.12.2.2 PARAXEL International Corporation
3.12.2.3 Bioclinica
3.12.2.4 Pharmaceutical Product Development (PPD)
3.12.2.5 IBM Corporation
3.12.2.6 ICON, plc
3.12.2.7 PRA Health Sciences
3.12.2.8 Covance Inc.
3.12.2.9 ArisGlobal
3.12.2.10 Linical Accelovance
3.12.2.11 Laboratory Corporation of America Holdings
3.13 Market Trends
3.13.1 Scaling Of Resources
3.13.2 Automation in Pharmacovigilance
3.14 Impact of Inflation
3.15 Comparative Analysis between Medical Writing vs Medical Safety Review
3.15.1 Medical Writing Market Outlook, 2022
3.15.1.1 Medical writing Market estimates and forecasts, 2018 - 2030 (USD Million)
3.15.2 Medical Safety Review Market Outlook, 2022
3.15.2.1 Medical Safety Review market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 4 Pharmacovigilance Market: Product Life Cycle Estimates & Trend Analysis
4.1 Product Life Cycle Market Share Analysis, 2022 & 2030
4.2 Product Life Cycle Dashboard
4.3 Market definition and scope
4.3.1 Preclinical
4.3.1.1 Preclinical market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.2 Phase I
4.3.2.1 Phase I market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.3 Phase II
4.3.3.1 Phase II market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.4 Phase III
4.3.4.1 Phase III market estimates and forecasts, 2018 - 2030 (USD Million)
4.3.5 Phase IV
4.3.5.1 Phase IV market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 5 Pharmacovigilance Market: Service Provider Estimates & Trend Analysis
5.1 Service provider Market Share Analysis, 2023 & 2030
5.2 Service Provider Dashboard
5.3 Market definition and scope
5.3.1 IN HOUSE
5.3.1.1 In house market estimates and forecasts, 2018 - 2030 (USD Million)
5.3.2 Contract Outsourcing
5.3.2.1 Contract outsourcing market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 6 Pharmacovigilance Market: Type Estimates & Trend Analysis
6.1 Type Market Share Analysis, 2023 & 2030
6.2 Type Dashboard
6.3 Market definition and scope
6.3.1 Spontaneous Reporting
6.3.1.1 Spontaneous Reporting market estimates and forecasts, 2018 - 2030 (USD Million)
6.3.2 Intensified ADR Reporting
6.3.2.1 Intensified ADR Reporting market estimates and forecasts, 2018 - 2030 (USD Million)
6.3.3 Targeted Spontaneous Reporting
6.3.3.1 Targeted spontaneous reporting market estimates and forecasts, 2018 - 2030 (USD Million)
6.3.4 Cohort Event Monitoring (CEM)
6.3.4.1 CEM market estimates and forecasts, 2018 - 2030 (USD Million)
6.3.5 EHR Mining
6.3.5.1 EHR Mining market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 7 Pharmacovigilance Market: Process Flow Estimates & Trend Analysis
7.1 Process Flow Market Share Analysis, 2023 & 2030
7.2 Process Flow Dashboard
7.3 Market definition and scope
7.3.1 Case Data Management
7.3.1.1 Case data management market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.1.1.1 Case logging
7.3.1.1.1.1 Case logging market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.1.1.2 Case data analysis
7.3.1.1.2.1 Case data analysis market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.1.1.3 Medical reviewing and reporting
7.3.1.1.3.1 Medical reviewing and reporting market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.2 Signal Detection
7.3.2.1 Signal detection market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.2.1.1 Adverse event logging
7.3.2.1.1.1 Adverse Event Logging market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.2.1.2 Adverse Event Analysis
7.3.2.1.2.1 Adverse Event Analysis market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.2.1.3 Adverse Event Review & Reporting
7.3.2.1.3.1 Adverse Event Review & Reporting market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.3 Risk Management System
7.3.3.1 Risk Management System market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.3.1.1 Risk Evaluation System
7.3.3.1.1.1 Risk Evaluation System market estimates and forecasts, 2018 - 2030 (USD Million)
7.3.3.1.2 Risk Mitigation System
7.3.2.1.2.1 Risk Mitigation System market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 8 Pharmacovigilance Market: Therapeutic Area Estimates & Trend Analysis
8.1 Therapeutic Area Market Share Analysis, 2023 & 2030
8.2 Therapeutic Area Dashboard
8.3 Market definition and scope
8.3.1 Oncology
8.3.1.1 Oncology market estimates and forecasts, 2018 - 2030 (USD Million)
8.3.2 Neurology
8.3.2.1 Neurology market estimates and forecasts, 2018 - 2030 (USD Million)
8.3.3 Cardiology
8.3.3.1 Cardiology market estimates and forecasts, 2018 - 2030 (USD Million)
8.3.4 Respiratory Systems
8.3.4.1 Respiratory systems market estimates and forecasts, 2018 - 2030 (USD Million)
8.3.5 Others
8.3.5.1 Others market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 9 Pharmacovigilance Market: End Use Estimates & Trend Analysis
9.1 End Use Market Share Analysis, 2023 & 2030
9.2 End Use Dashboard
9.3 Market definition and scope
9.3.1 Pharmaceuticals
9.3.1.1 Pharmaceuticals market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.2 Biotechnology Companies
9.3.2.1 Biotechnology companies market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.3 Medical Device Manufacturers
9.3.3.1 Medical Device Manufacturers market estimates and forecasts, 2018 - 2030 (USD Million)
9.3.4 Others
9.3.4.1 Others market estimates and forecasts, 2018 - 2030 (USD Million)
Chapter 10 Pharmacovigilance Market: Regional Estimates & Trend Analysis, By, Product, Service Providers, Type, and End Use
10.1 Regional Market Snapshot
10.2 North America
10.2.1 North America Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.2.2 U.S.
10.2.2.1 U.S. Pharmacovigilance market estimates and forecasts, 2018- 2030 (USD Million)
10.2.2.2 Competitive scenario
10.2.2.3 Regulatory Framework
10.2.3 Canada
10.2.3.1 Canada Pharmacovigilance market estimates and Forecasts, 2018 - 2030 (USD Million)
10.2.3.2 Competitive scenario
10.2.3.3 Regulatory Framework
10.3 Europe
10.3.1 Europe Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.3.2 UK
10.3.2.1 UK Pharmacovigilance market estimates and Forecasts, 2018 - 2030 (USD Million)
10.3.2.2 Competitive scenario
10.3.2.3 Regulatory Framework
10.3.3 Germany
10.3.3.1 Germany Pharmacovigilance market estimates and forecasts, 2018 - 2030 (USD Million
10.3.3.2 Competitive scenario
10.3.3.3 Regulatory Framework
10.3.4 France
10.3.4.1 France Pharmacovigilance market estimates and forecasts, 2018- 2030 (USD Million)
10.3.4.2 Competitive scenario
10.3.4.3 Regulatory Framework
10.3.5 Italy
10.3.5.1 Italy Pharmacovigilance market estimates and forecasts, 2018 - 2030 (USD Million)
10.3.5.2 Competitive scenario
10.3.5.3 Regulatory Framework
10.3.6 Spain
10.3.6.1 Spain pharmacovigilance market estimates and forecasts, 2018 - 2030 (USD Million)
10.3.6.2 Competitive scenario
10.3.6.3 Regulatory Framework
10.3.7 Russia
10.3.7.1 Russia Pharmacovigilance market estimates and forecasts, 2018- 2030 (USD Million)
10.3.7.2 Competitive scenario
10.3.7.3 Regulatory Framework
10.3.8 Denmark
10.3.8.1 Denmark Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.3.8.2 Competitive scenario
10.3.8.3 Regulatory Framework
10.3.9 Norway
10.3.9.1 Norway Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.3.9.2 Competitive scenario
10.3.9.3 Regulatory Framework
10.3.10 Sweden
10.3.10.1 Sweden Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.3.10.2 Competitive scenario
10.3.10.3 Regulatory Framework
10.4 Asia Pacific
10.4.1 Asia Pacific Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.4.2 JAPAN
10.4.2.1 Japan Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.4.2.2 Competitive scenario
10.4.2.3 Regulatory Framework
10.4.3 CHINA
10.4.3.1 China Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.4.3.2 Competitive Scenario
10.4.3.3 Regulatory Framework
10.4.4 India
10.4.4.1 India Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.4.4.2 Competitive Scenario
10.4.4.3 Regulatory Framework
10.4.5 Australia
10.4.5.1 Australia Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.4.5.2 Competitive Scenario
10.4.5.3 Regulatory Framework
10.4.6 Thailand
10.4.6.1 Thailand Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.4.6.2 Competitive Scenario
10.4.6.3 Regulatory Framework
10.4.7 South Korea
10.4.7.1 South Korea Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.4.7.2 Competitive Scenario
10.4.7.3 Regulatory Framework
10.5 Latin America
10.5.1 Latin America Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.5.2 Brazil
10.5.2.1 Brazil Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.5.2.2 Competitive Scenario
10.5.2.3 Regulatory Framework
10.5.3 Mexico
10.5.3.1 Mexico Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.5.3.2 Competitive Scenario
10.5.3.3 Regulatory Framework
10.5.4 Argentina
10.5.4.1 Argentina Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.5.4.2 Competitive Scenario
10.5.4.3 Regulatory Framework
10.6 MEA
10.6.1 MEA Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.6.2 South Africa
10.6.2.1 South Africa Pharmacovigilance Market Estimates and Forecasts, 2018 - 2030 (USD Million)
10.6.2.2 Competitive Scenario
10.6.2.3 Regulatory Framework
10.6.3 Saudi Arabia
10.6.3.1 Saudi Arabia Pharmacovigilance Market Estimates and Forecasts, 2018- 2030 (USD Million)
10.6.3.2 Competitive Scenario
10.6.3.3 Regulatory Framework
10.6.4 UAE
10.6.4.1 UAE Arabia Pharmacovigilance Market Estimates and Forecasts, 2018- 2030 (USD Million)
10.6.4.2 Competitive Scenario
10.6.4.3 Regulatory Framework
10.6.5 Kuwait
10.6.5.1 Kuwait Pharmacovigilance Market Estimates and Forecasts, 2018- 2030 (USD Million)
10.6.5.2 Competitive Scenario
10.6.5.3 Regulatory Framework
Chapter 11 Pharmacovigilance Market: Competitive Analysis
11.1 Market Participation Categorization
11.2 Public Companies
11.2.1 Company Market Position Analysis
11.2.2 Company Market Share
11.3 Private Companies
11.3.1 List of Key Emerging Companies
11.4 Competitive Factors and Strategies
11.4.1 Increasing strategic collaborations and product launch
11.4.2 Strategic Government initiatives which include collaborations
11.4.3 Competitors increased PV awareness program
11.4.4 Competitors increased collaboration and outsourcing of operations
11.4.5 Consolidation Trends
11.5 Healthcare Companies Using Pharmacovigilance Services
11.5.1 Potential Customers
11.5.2 Notable Cases
11.5.2.1 Case Study 1
11.5.2.1.1 Case Study 1
11.5.3.1.2 Case Study 2
11.5.2.2 Case Study 2
11.5.2.3 Case Study 3
11.5.3.4 Case Study 4
11.5.3 Key Focus areas for the pharmaceutical/healthcare companies
11.5.3.1 Adoption of technological advancements in life sciences industry
11.5.3.2 Published survey insights - related to adoption of pharmacovigilance automation
Chapter 12 Competitive Landscape
12.1 Company Profiles
12.1.1 ACCENTURE
12.1.1.1 Company overview
12.1.1.2 Service benchmarking
12.1.1.3 Financial performance
12.1.1.4 Strategic initiatives
12.1.1.5 SWOT Analysis
12.1.2 CLINQUEST GROUP B.V. (LINICAL AMERICAS)
12.1.2.1 Company overview
12.1.2.2 Service benchmarking
12.1.2.3 Financial performance
12.1.2.4 Strategic initiatives
12.1.2.5 SWOT Analysis
12.1.3 IQVIA Inc.
12.1.3.1 Company overview
12.1.3.2 Service benchmarking
12.1.3.3 Financial performance
12.1.3.4 Strategic initiatives
12.1.3.5 SWOT Analysis
12.1.4 COGNIZANT
12.1.4.1 Company overview
12.1.4.2 Service benchmarking
12.1.4.3 Financial performance
12.1.4.4 Strategic initiatives
12.1.4.5 SWOT Analysis
12.1.5 LABORATORY CORPORATION OF AMERICA HOLDINGS
12.1.5.1 Company overview
12.1.5.2 Service benchmarking
12.1.5.3 Financial performance
12.1.5.4 Strategic initiatives
12.1.5.5 SWOT Analysis
12.1.6 IBM
12.1.6.1 Company overview
12.1.6.2 Service benchmarking
12.1.6.3 Financial performance
12.1.6.4 Strategic initiatives
12.1.6.5 SWOT Analysis
12.1.7 ARISGLOBAL
12.1.7.1 Company Overview
12.1.7.2 Service Benchmarking
12.1.7.3 Financial Performance
12.1.7.4 Strategic Initiatives
12.1.7.5 SWOT Analysis
12.1.8 ICON PLC.
12.1.8.1 Company Overview
12.1.8.2 Service Benchmarking
12.1.8.3 Financial Performance
12.1.8.4 Strategic Initiatives
12.1.8.5 SWOT Analysis
12.1.9 CAPGEMINI
12.1.9.1 Company Overview
12.1.9.2 Service Benchmarking
12.1.9.3 Financial Performance
12.1.9.4 Strategic Initiatives
12.1.9.5 SWOT Analysis
12.1.10 ITCLINICAL
12.1.10.1 Company Overview
12.1.10.2 Service Benchmarking
12.1.10.3 Financial Performance
12.1.10.4 Strategic Initiatives
12.1.10.5 SWOT Analysis
12.1.11 TAKE SOLUTIONS LIMITED
12.1.11.1 Company Overview
12.1.11.2 Service Benchmarking
12.1.11.3 Financial Performance
12.1.11.4 Strategic Initiatives
12.1.11.5 SWOT Analysis
12.1.12 PAREXEL INTERNATIONAL CORPORATION.
12.1.12.1 Company Overview
12.1.12.2 Service Benchmarking
12.1.12.3 Financial Performance
12.1.12.4 Strategic Initiatives
12.1.12.5 SWOT Analysis
12.1.13 BIOCLINICA, INC.
12.1.13.1 Company overview
12.1.13.2 Service benchmarking
12.1.13.3 Financial performance
12.1.13.4 Strategic initiatives
12.1.13.5 SWOT Analysis
12.1.14 WIPRO
12.1.14.1 Company Overview
12.1.14.2 Service Benchmarking
12.1.14.3 Financial Performance
12.1.14.4 Strategic Initiatives
12.1.14.5 SWOT Analysis
12.1.15 UNITED BIOSOURCE LLC
12.1.15.1 Company Overview
12.1.15.2 Service Benchmarking
12.1.15.3 Financial Performance
12.1.15.4 Strategic initiatives
12.1.15.5 SWOT Analysis
12.1.16 FMD K&L (CLINCHOICE)
12.1.16.1 Company Overview
12.1.16.2 Service Benchmarking
12.1.16.3 Financial performance
12.1.16.4 Strategic initiatives
12.1.16.5 SWOT Analysis
Chapter 13 Winning Strategies
13.1 Key Winning/Scoring Criteria
13.1.1 By Categories
13.1.1.1 Pharmaceuticals
13.1.1.2 Biotech companies
13.1.1.3 Medical device companies
13.2 Key Vendor Selection Factors
13.2.1 By Category
13.2.2 By Company Size
13.2.2.1 Key Takeaways
Chapter 14 Switching Cost Analysis
List of Tables
Table 1 List of secondary sources
Table 2 List of Abbreviation
Table 3 Types of ADRs
Table 4 Adverse Drug Events (ADEs) in hospitals
Table 5 Pharmacovigilance outsourcing services insights
Table 6 List of regulations, by country
Table 7 Estimated Total Per-Study Costs (in $ Millions), by Phase and Therapeutic Area
Table 8 Case management costing by year
Table 9 Price by case processing volume
Table 10 Subscription services for medical manuscript writing
Table 11 Total literature searches (2013-2022)
Table 12 Instances of failed phase I clinical trials for COVID-19 vaccine
Table 13 Recruiting phase II clinical trials for oncology
Table 14 Outsourcing trend observed in pharmaceutical companies
Table 15 Recent Market Events
Table 16 List of Major Deals & Acquisitions
Table 17 List of key distributors and channel partners
Table 18 List of key emerging companies’/technology disruptors/innovators
Table 19 North America Pharmacovigilance Market, by country, 2018 - 2030 (USD Million)
Table 20 North America Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 21 North America Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 22 North America Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 23 U.S. Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 24 U.S. Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 25 U.S. Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 26 Canada Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 27 Canada Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 28 Canada Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 29 Europe Pharmacovigilance Market, by country, 2018 - 2030 (USD Million)
Table 30 Europe Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 31 Europe Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 32 Europe Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 33 Germany Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 34 Germany Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 35 Germany Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 36 UK Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 37 UK Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 38 UK Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 39 France Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 40 France Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 41 France Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 42 Italy Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 43 Italy Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 44 Italy Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 45 Spain Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 46 Spain Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 47 Sapin Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 48 Denmark Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 49 Denmark Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 50 Denmark Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 51 Sweden Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 52 Sweden Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 53 Sweden Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 54 Norway Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 55 Norway Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 56 Norway Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 57 Asia Pacific Pharmacovigilance Market, by country, 2018 - 2030 (USD Million)
Table 58 Asia Pacific Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 59 Asia Pacific Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 60 Asia Pacific Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 61 Japan Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 62 Japan Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 63 Japan Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 64 China Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 65 China Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 66 China Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 67 India Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 68 India Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 69 India Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 70 Australia Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 71 Australia Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 72 Australia Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 73 South Korea Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 74 Thailand Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 75 Thailand Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 76 Thailand Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 77 Latin America Pharmacovigilance Market, by country, 2018 - 2030 (USD Million)
Table 78 Latin America Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 79 Latin America Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 80 Latin America Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 81 Brazil Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 82 Brazil Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 83 Brazil Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 84 Mexico Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 85 Mexico Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 86 Mexico Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 87 Argentina Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 88 Argentina Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 89 Argentina Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 90 MEA Pharmacovigilance Market, by country, 2018 - 2030 (USD Million)
Table 91 MEA Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 92 MEA Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 93 MEA Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 94 South Africa Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 95 South Africa Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 96 South Africa Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 97 Saudi Arabia Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 98 Saudi Arabia Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 99 Saudi Arabia Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 100 UAE Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 101 UAE Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 102 UAE Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
Table 103 Kuwait Pharmacovigilance Market, by Injection Type, 2018 - 2030 (USD Million)
Table 104 Kuwait Pharmacovigilance Market, by Anatomy, 2018 - 2030 (USD Million)
Table 105 Kuwait Pharmacovigilance Market, by End Use, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Pharmacovigilance market segmentation
Fig. 2 Market research process
Fig. 3 Data triangulation techniques
Fig. 4 Primary research pattern
Fig. 5 Primary interviews in North America
Fig. 6 Primary interviews in Europe
Fig. 7 Primary interviews in APAC
Fig. 8 Primary interviews in Latin America
Fig. 9 Primary interviews in MEA
Fig. 10 Market research approaches
Fig. 11 Value-chain-based sizing & forecasting
Fig. 12 QFD modeling for market share assessment
Fig. 13 Market formulation & validation
Fig. 14 Timeline of pharmacovigilance for a drug from development (premarket to post marketing)
Fig. 15 Market Snapshot
Fig. 16 Segment Snapshot (Product Life Cycle & Service Provider)
Fig. 17 Segment Snapshot (Type & Therapeutic Area)
Fig. 18 Segment Snapshot (Process Flow & End Use)
Fig. 19 Competitive Landscape Snapshot
Fig. 20 Penetration & growth prospect mapping
Fig. 21 Pharmacovigilance market dynamics
Fig. 22 Porter’s five force model
Fig. 23 Pharmacovigilance - SWOT analysis, by factor (political & legal, economic, and technological)
Fig. 24 Pharmacovigilance & Patient Safety Services - Product Life Cycle
Fig. 25 PV department
Fig. 26 PV organization structure
Fig. 27 Estimated budget allocation of drug safety activities at global and country-level
Fig. 28 Comparison between estimated global and country-level drug safety budget allocation
Fig. 29 Average % of drug safety budget contributed by function at a global level
Fig. 30 Pharmacovigilance system cost for small-sized firms and larger-sized firms
Fig. 31 Clinical trial cost by phase (%)
Fig. 32 Selected clinical trials costs based on technology
Fig. 33 Estimated cost of developing therapeutic medical device in the U.S.
Fig. 34 The average monthly cost of illness due to ADRs
Fig. 35 Medical Writers Employers by Industry
Fig. 36 Trending topics on social media (2017)
Fig. 37 ADR detection from social media data
Fig. 38 Common literature automation tool flow
Fig. 39 Automation processes in PV
Fig. 40 Deterrents to Leveraging the Cloud (Oracle Survey)
Fig. 41 The three-step process to compute signal statistics from search log using big data
Fig. 42 Risk Management Capacity & Capability with Pharmacovigilance Using Big Data
Fig. 43 Pharma & Biotech R&D expense growth, 2020 (%)
Fig. 44 Medical writing market outlook, 2022 (USD Million)
Fig. 45 Medical writing market, 2018 - 2030 (USD Million)
Fig. 46 North America medical safety review market, 2018 - 2030 (USD Million)
Fig. 47 North America Pharmacovigilance market: Product life cycle movement analysis
Fig. 48 North America Pharmacovigilance market product life cycle dashboard
Fig. 49 North America Preclinical market, 2018 - 2030 (USD Million)
Fig. 50 North America Phase I market, 2018 - 2030 (USD Million)
Fig. 51 North America Phase II market, 2018 - 2030 (USD Million)
Fig. 52 North America Phase III market, 2018 - 2030 (USD Million)
Fig. 53 Adverse events reported in FAERS system, 2019-2022
Fig. 54 North America Phase IV market, 2018 - 2030 (USD Million)
Fig. 55 North America Pharmacovigilance market: Service provider movement analysis
Fig. 56 North America Pharmacovigilance Service provider dashboard
Fig. 57 North America In-house market, 2018 - 2030 (USD Million)
Fig. 58 North America contract outsourcing market, 2018 - 2030 (USD Million)
Fig. 59 North America Pharmacovigilance market: Type movement analysis
Fig. 60 North America Pharmacovigilance type dashboard
Fig. 61 North America spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 62 North America intensified ADR reporting market, 2018 - 2030 (USD Million)
Fig. 63 North America targeted spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 64 North America CEM market, 2018 - 2030 (USD Million)
Fig. 65 North America EHR mining market, 2018 - 2030 (USD Million)
Fig. 66 North America Pharmacovigilance market: Process flow movement analysis
Fig. 67 North America Pharmacovigilance market process flow dashboard
Fig. 68 North America case data management market, 2018 - 2030 (USD Million)
Fig. 69 North America case logging market, 2018 - 2030 (USD Million)
Fig. 70 North America case data analysis market, 2018 - 2030 (USD Million)
Fig. 71 North America medical reviewing and reporting market, 2018 - 2030 (USD Million)
Fig. 72 North America signal detection market, 2018 - 2030 (USD Million)
Fig. 73 North America adverse event logging market, 2018 - 2030 (USD Million)
Fig. 74 North America adverse event analysis market, 2018 - 2030 (USD Million)
Fig. 75 North America adverse event review & reporting market, 2018 - 2030 (USD Million)
Fig. 76 North America risk management system market, 2018 - 2030 (USD Million)
Fig. 77 North America Risk Evaluation System market, 2018 - 2030 (USD Million)
Fig. 78 North America risk mitigation system market, 2018 - 2030 (USD Million)
Fig. 79 North America Pharmacovigilance market: Therapeutic area movement analysis
Fig. 80 North America Pharmacovigilance market therapeutic area dashboard
Fig. 81 North America oncology market, 2018 - 2030 (USD Million)
Fig. 82 North America neurology market, 2018 - 2030 (USD Million)
Fig. 83 North America cardiology market, 2018 - 2030 (USD Million)
Fig. 84 North America respiratory systems market, 2018 - 2030 (USD Million)
Fig. 85 North America others market, 2018 - 2030 (USD Million)
Fig. 86 North America Pharmacovigilance market: End use movement analysis
Fig. 87 North America Pharmacovigilance market end use dashboard
Fig. 88 North America pharmaceuticals market, 2018 - 2030 (USD Million)
Fig. 89 North America biotechnology companies’ market, 2018 - 2030 (USD Million)
Fig. 90 North America medical device manufacturers market, 2018 - 2030 (USD Million)
Fig. 91 North America others market, 2018 - 2030 (USD Million)
Fig. 92 Country market place: Key takeaways
Fig. 93 U.S. key country dynamics (Part 1)
Fig. 94 U.S. key country dynamics (Part 2)
Fig. 95 U.S. pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 96 Canada key country dynamics (Part 1)
Fig. 97 Canada key country dynamics (Part 2)
Fig. 98 Canada pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 99 Europe medical safety review market, 2018 - 2030 (USD Million)
Fig. 100 Europe Pharmacovigilance market: Product life cycle movement analysis
Fig. 101 Europe Pharmacovigilance market product life cycle dashboard
Fig. 102 Europe Preclinical market, 2018 - 2030 (USD Million)
Fig. 103 Europe Phase I market, 2018 - 2030 (USD Million)
Fig. 104 Europe Phase II market, 2018 - 2030 (USD Million)
Fig. 105 Europe Phase III market, 2018 - 2030 (USD Million)
Fig. 106 Adverse events reported in FAERS system, 2019-2022
Fig. 107 Europe Phase IV market, 2018 - 2030 (USD Million)
Fig. 108 Europe Pharmacovigilance market: Service provider movement analysis
Fig. 109 Europe Pharmacovigilance Service provider dashboard
Fig. 110 Europe In-house market, 2018 - 2030 (USD Million)
Fig. 111 Europe contract outsourcing market, 2018 - 2030 (USD Million)
Fig. 112 Europe Pharmacovigilance market: Type movement analysis
Fig. 113 Europe Pharmacovigilance type dashboard
Fig. 114 Europe spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 115 Europe intensified ADR reporting market, 2018 - 2030 (USD Million)
Fig. 116 Europe targeted spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 117 Europe CEM market, 2018 - 2030 (USD Million)
Fig. 118 Europe EHR mining market, 2018 - 2030 (USD Million)
Fig. 119 Europe Pharmacovigilance market: Process flow movement analysis
Fig. 120 Europe Pharmacovigilance market process flow dashboard
Fig. 121 Europe case data management market, 2018 - 2030 (USD Million)
Fig. 122 Europe case logging market, 2018 - 2030 (USD Million)
Fig. 123 Europe case data analysis market, 2018 - 2030 (USD Million)
Fig. 124 Europe medical reviewing and reporting market, 2018 - 2030 (USD Million)
Fig. 125 Europe signal detection market, 2018 - 2030(USD Million)
Fig. 126 Europe adverse event logging market, 2018 - 2030 (USD Million)
Fig. 127 Europe adverse event analysis market, 2018 - 2030 (USD Million)
Fig. 128 Europe adverse event review & reporting market, 2018 - 2030 (USD Million)
Fig. 129 Europe risk management system market, 2018 - 2030 (USD Million)
Fig. 130 Europe Risk Evaluation System market, 2018 - 2030 (USD Million)
Fig. 131 Europe risk mitigation system market, 2018 - 2030 (USD Million)
Fig. 132 Europe Pharmacovigilance market: Therapeutic area movement analysis
Fig. 133 Europe Pharmacovigilance market therapeutic area dashboard
Fig. 134 Europe oncology market, 2018 - 2030 (USD Million)
Fig. 135 Europe neurology market, 2018 - 2030 (USD Million)
Fig. 136 Europe cardiology market, 2018 - 2030 (USD Million)
Fig. 137 Europe respiratory systems market, 2018 - 2030 (USD Million)
Fig. 138 Europe others market, 2018 - 2030 (USD Million)
Fig. 139 Europe Pharmacovigilance market: End use movement analysis
Fig. 140 Europe Pharmacovigilance market end use dashboard
Fig. 141 Europe pharmaceuticals market, 2018 - 2030 (USD Million)
Fig. 142 Europe biotechnology companies’ market, 2018 - 2030 (USD Million)
Fig. 143 Europe medical device manufacturers market, 2018 - 2030 (USD Million)
Fig. 144 Europe others market, 2018 - 2030 (USD Million)
Fig. 145 Country market place: Key takeaways
Fig. 146 UK key country dynamics (Part 1)
Fig. 147 UK key country dynamics (Part 2)
Fig. 148 UK pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 149 Germany key country dynamics (Part 1)
Fig. 150 Germany key country dynamics (Part 2)
Fig. 151 Germany pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 152 France key country dynamics (Part 1)
Fig. 153 France key country dynamics (Part 2)
Fig. 154 France pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 155 Italy key country dynamics (Part 1)
Fig. 156 Italy key country dynamics (Part 2)
Fig. 157 Italy pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 158 Spain key country dynamics (Part 1)
Fig. 159 Spain key country dynamics (Part 2)
Fig. 160 Spain pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 161 Russia key country dynamics
Fig. 162 Russia pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 163 Denmark key country dynamics (Part 1)
Fig. 164 Denmark key country dynamics (Part 2)
Fig. 165 Denmark pharmacovigilance market estimates and forecasts, 2018 - 2030, (USD Million)
Fig. 166 Norway key country dynamics (Part 1)
Fig. 167 Norway key country dynamics (Part 2)
Fig. 168 Norway pharmacovigilance market estimates and forecasts, 2018 - 2030, (USD Million)
Fig. 169 Sweden key country dynamics (Part 1)
Fig. 170 Sweden key country dynamics (Part 2)
Fig. 171 Sweden pharmacovigilance market estimates and forecasts, 2018 - 2030, (USD Million)
Fig. 170 Sweden key country dynamics (Part 2)
Fig. 171 Sweden pharmacovigilance market estimates and forecasts, 2018 - 2030, (USD Million)
Fig. 172 Latin America medical safety review market, 2018 - 2030 (USD Million)
Fig. 173 Latin America Pharmacovigilance market: Product life cycle movement analysis
Fig. 174 Latin America Pharmacovigilance market product life cycle dashboard
Fig. 175 Latin America Preclinical market, 2018 - 2030 (USD Million)
Fig. 176 Latin America Phase I market, 2018 - 2030 (USD Million)
Fig. 177 Latin America Phase II market, 2018 - 2030 (USD Million)
Fig. 178 Latin America Phase III market, 2018 - 2030 (USD Million)
Fig. 179 Latin America Phase IV market, 2018 - 2030 (USD Million)
Fig. 180 Latin America Pharmacovigilance market: Service provider movement analysis
Fig. 181 Latin America Pharmacovigilance Service provider dashboard
Fig. 182 Latin America In-house market, 2018 - 2030 (USD Million)
Fig. 183 Latin America contract outsourcing market, 2018 - 2030 (USD Million)
Fig. 184 Latin America Pharmacovigilance market: Type movement analysis
Fig. 185 Latin America Pharmacovigilance type dashboard
Fig. 186 Latin America spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 187 Latin America intensified ADR reporting market, 2018 - 2030 (USD Million)
Fig. 188 Latin America targeted spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 189 Latin America CEM market, 2018 - 2030 (USD Million)
Fig. 190 Latin America EHR mining market, 2018 - 2030 (USD Million)
Fig. 191 Latin America Pharmacovigilance market: Process flow movement analysis
Fig. 192 Latin America Pharmacovigilance market process flow dashboard
Fig. 193 Latin America case data management market, 2018 - 2030 (USD Million)
Fig. 194 Latin America case logging market, 2018 - 2030 (USD Million)
Fig. 195 Latin America case data analysis market, 2018 - 2030 (USD Million)
Fig. 196 Latin America medical reviewing and reporting market, 2018 - 2030 (USD Million)
Fig. 197 Latin America signal detection market, 2018 - 2030 (USD Million)
Fig. 198 Latin America adverse event logging market, 2018 - 2030 (USD Million)
Fig. 199 Latin America adverse event analysis market, 2018 - 2030 (USD Million)
Fig. 200 Latin America adverse event review & reporting market, 2018 - 2030 (USD Million)
Fig. 201 Latin America risk management system market, 2018 - 2030 (USD Million)
Fig. 202 Latin America Risk Evaluation System market, 2018 - 2030 (USD Million)
Fig. 203 Latin America risk mitigation system market, 2018 - 2030 (USD Million)
Fig. 204 Latin America Pharmacovigilance market: Therapeutic area movement analysis
Fig. 205 Latin America Pharmacovigilance market therapeutic area dashboard
Fig. 206 Latin America oncology market, 2018 - 2030 (USD Million)
Fig. 207 Latin America neurology market, 2018 - 2030 (USD Million)
Fig. 208 Latin America cardiology market, 2018 - 2030 (USD Million)
Fig. 209 Latin America respiratory systems market, 2018 - 2030 (USD Million)
Fig. 210 Latin America others market, 2018 - 2030 (USD Million)
Fig. 211 Latin America Pharmacovigilance market: End use movement analysis
Fig. 212 Latin America Pharmacovigilance market end use dashboard
Fig. 213 Latin America pharmaceuticals market, 2018 - 2030 (USD Million)
Fig. 214 Latin America biotechnology companies’ market, 2018 - 2030 (USD Million)
Fig. 215 Latin America medical device manufacturers market, 2018 - 2030 (USD Million)
Fig. 216 Latin America others market, 2018 - 2030 (USD Million)
Fig. 217 Brazil key country dynamics (Part 1)
Fig. 218 Brazil key country dynamics (Part 2)
Fig. 219 Brazil pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 220 Mexico key country dynamics (Part 1)
Fig. 221 Mexico key country dynamics (Part 2)
Fig. 222 Mexico pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 223 Argentina key country dynamics (Part 1)
Fig. 224 Argentina key country dynamics (Part 2)
Fig. 225 Argentina pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 226 MEA medical safety review market, 2018 - 2030 (USD Million)
Fig. 227 MEA Pharmacovigilance market: Product life cycle movement analysis
Fig. 228 MEA Pharmacovigilance market product life cycle dashboard
Fig. 229 MEA Preclinical market, 2018 - 2030 (USD Million)
Fig. 230 MEA Phase I market, 2018 - 2030 (USD Million)
Fig. 231 MEA Phase II market, 2018 - 2030 (USD Million)
Fig. 232 MEA Phase III market, 2018 - 2030 (USD Million)
Fig. 233 MEA Phase IV market, 2018 - 2030 (USD Million)
Fig. 234 MEA Pharmacovigilance market: Service provider movement analysis
Fig. 235 MEA Pharmacovigilance Service provider dashboard
Fig. 236 MEA In-house market, 2018 - 2030 (USD Million)
Fig. 237 MEA contract outsourcing market, 2018 - 2030 (USD Million)
Fig. 238 MEA Pharmacovigilance market: Type movement analysis
Fig. 239 MEA Pharmacovigilance type dashboard
Fig. 240 MEA spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 241 MEA intensified ADR reporting market, 2018 - 2030 (USD Million)
Fig. 242 MEA targeted spontaneous reporting market, 2018 - 2030 (USD Million)
Fig. 243 MEA CEM market, 2018 - 2030 (USD Million)
Fig. 244 MEA EHR mining market, 2018 - 2030 (USD Million)
Fig. 245 MEA Pharmacovigilance market: Process flow movement analysis
Fig. 246 MEA Pharmacovigilance market process flow dashboard
Fig. 247 MEA case data management market, 2018 - 2030 (USD Million)
Fig. 248 MEA case logging market, 2018 - 2030 (USD Million)
Fig. 249 MEA case data analysis market, 2018 - 2030 (USD Million)
Fig. 250 MEA medical reviewing and reporting market, 2018 - 2030 (USD Million)
Fig. 251 MEA signal detection market, 2018 - 2030 (USD Million)
Fig. 252 MEA adverse event logging market, 2018 - 2030 (USD Million)
Fig. 253 MEA adverse event analysis market, 2018 - 2030 (USD Million)
Fig. 254 MEA adverse event review & reporting market, 2018 - 2030 (USD Million)
Fig. 255 MEA risk management system market, 2018 - 2030 (USD Million)
Fig. 256 MEA Risk Evaluation System market, 2018 - 2030 (USD Million)
Fig. 257 MEA risk mitigation system market, 2018 - 2030 (USD Million)
Fig. 258 MEA Pharmacovigilance market: Therapeutic area movement analysis
Fig. 259 MEA Pharmacovigilance market therapeutic area dashboard
Fig. 260 MEA oncology market, 2018 - 2030 (USD Million)
Fig. 261 MEA neurology market, 2018 - 2030 (USD Million)
Fig. 262 MEA cardiology market, 2018 - 2030 (USD Million)
Fig. 263 MEA respiratory systems market, 2018 - 2030 (USD Million)
Fig. 264 MEA others market, 2018 - 2030 (USD Million)
Fig. 265 MEA Pharmacovigilance market: End use movement analysis
Fig. 266 MEA Pharmacovigilance market end use dashboard
Fig. 267 MEA pharmaceuticals market, 2018 - 2030 (USD Million)
Fig. 268 MEA biotechnology companies’ market, 2018 - 2030 (USD Million)
Fig. 269 MEA medical device manufacturers market, 2018 - 2030 (USD Million)
Fig. 270 MEA others market, 2018 - 2030 (USD Million)
Fig. 271 South Africa key country dynamics (Part 1)
Fig. 272 South Africa key country dynamics (Part 2)
Fig. 273 South Africa pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 274 Saudi Arabia key country dynamics (Part 1)
Fig. 275 Saudi Arabia key country dynamics (Part 2)
Fig. 276 Saudi Arabia pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 277 UAE key country dynamics (Part 1)
Fig. 278 UAE key country dynamics (Part 2)
Fig. 279 UAE pharmacovigilance market, 2018 - 2030 (USD Million)
Fig. 280 Kuwait key country dynamics (Part 1)
Fig. 281 Kuwait key country dynamics (Part 2)
Fig. 282 Kuwait pharmacovigilance market, 2018 - 2030 (USD Million)What questions do you have? Get quick response from our industry experts. Request a Free ConsultationMarket Segmentation
- Pharmacovigilance Product Life Cycle Outlook (Revenue, USD Million, 2018 - 2030)
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Pharmacovigilance Service Provider Outlook (Revenue, USD Million, 2018 - 2030)
- In-house
- Contract Outsourcing
- Pharmacovigilance Type Outlook (Revenue, USD Million, 2018 - 2030)
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Pharmacovigilance Process Flow Outlook (Revenue, USD Million, 2018 - 2030)
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Pharmacovigilance Therapeutic Area Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Pharmacovigilance End Use Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Pharmacovigilance Regional Outlook (USD Million,2018 - 2030)
- North America
- North America Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- North America Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- North America Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- North America Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- North America Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- North America Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- U.S.
- U.S. Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- U.S. Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- U.S. Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- U.S. Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- U.S. Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- U.S. Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- U.S. Pharmacovigilance Market, by Product Life Cycle
- Canada
- Canada Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Canada Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Canada Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Canada Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Canada Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Canada Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Canada Pharmacovigilance Market, by Product Life Cycle
- North America Pharmacovigilance Market, by Product Life Cycle
- Europe
- Europe Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Europe Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Europe Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Europe Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Europe Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Europe Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- UK
- UK Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- UK Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- UK Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- UK Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- UK Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- UK Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- UK Pharmacovigilance Market, by Product Life Cycle
- Germany
- Germany Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Germany Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Germany Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Germany Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Germany Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Germany Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Germany Pharmacovigilance Market, by Product Life Cycle
- France
- France Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- France Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- France Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- France Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- France Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- France Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- France Pharmacovigilance Market, by Product Life Cycle
- Italy
- Italy Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Italy Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Italy Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Italy Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Italy Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Italy Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Italy Pharmacovigilance Market, by Product Life Cycle
- Spain
- Spain Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Spain Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Spain Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Spain Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Spain Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Spain Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Spain Pharmacovigilance Market, by Product Life Cycle
- Denmark
- Denmark Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Denmark Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Denmark Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Denmark Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Denmark Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Denmark Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Denmark Pharmacovigilance Market, by Product Life Cycle
- Sweden
- Sweden Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Sweden Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Sweden Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Sweden Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Sweden Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Sweden Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Sweden Pharmacovigilance Market, by Product Life Cycle
- Norway
- Norway Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Norway Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Norway Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Norway Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Norway Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Norway Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Norway Pharmacovigilance Market, by Product Life Cycle
- Russia
- Russia Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Russia Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Russia Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Russia Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Russia Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Russia Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Russia Pharmacovigilance Market, by Product Life Cycle
- Rest of Europe
- Rest of Europe Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Rest of Europe Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Rest of Europe Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Rest of Europe Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Rest of Europe Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Rest of Europe Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Rest of Europe Pharmacovigilance Market, by Product Life Cycle
- Europe Pharmacovigilance Market, by Product Life Cycle
- Asia Pacific
- Asia Pacific Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Asia Pacific Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Asia Pacific Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Asia Pacific Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Asia Pacific Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Asia Pacific Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Japan
- Japan Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Japan Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Japan Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Japan Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Japan Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Japan Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Japan Pharmacovigilance Market, by Product Life Cycle
- China
- China Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- China Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- China Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- China Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- China Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- China Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- China Pharmacovigilance Market, by Product Life Cycle
- India
- India Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- India Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- India Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- India Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- India Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- India Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- India Pharmacovigilance Market, by Product Life Cycle
- Australia
- Australia Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Australia Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Australia Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Australia Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Australia Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Australia Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Australia Pharmacovigilance Market, by Product Life Cycle
- Thailand
- Thailand Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Thailand Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Thailand Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Thailand Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Thailand Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Thailand Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Thailand Pharmacovigilance Market, by Product Life Cycle
- South Korea
- South Korea Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- South Korea Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- South Korea Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- South Korea Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- South Korea Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- South Korea Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- South Korea Pharmacovigilance Market, by Product Life Cycle
- Rest of Asia Pacific
- Rest of Asia Pacific Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Rest of Asia Pacific Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Rest of Asia Pacific Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Rest of Asia Pacific Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Rest of Asia Pacific Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Rest of Asia Pacific Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Rest of Asia Pacific Pharmacovigilance Market, by Product Life Cycle
- Asia Pacific Pharmacovigilance Market, by Product Life Cycle
- Latin America
- Latin America Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Latin America Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Latin America Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Latin America Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Latin America Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Latin America Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Brazil
- Brazil Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Brazil Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Brazil Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Brazil Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Brazil Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Brazil Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Brazil Pharmacovigilance Market, by Product Life Cycle
- Mexico
- Mexico Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Mexico Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Mexico Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Mexico Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Mexico Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Mexico Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Mexico Pharmacovigilance Market, by Product Life Cycle
- Argentina
- Argentina Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Argentina Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Argentina Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Argentina Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Argentina Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Argentina Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Argentina Pharmacovigilance Market, by Product Life Cycle
- Rest of Latin America
- Rest of Latin America Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Rest of Latin America Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Rest of Latin America Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Rest of Latin America Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Rest of Latin America Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Rest of Latin America Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Rest of Latin America Pharmacovigilance Market, by Product Life Cycle
- Latin America Pharmacovigilance Market, by Product Life Cycle
- The Middle East and Africa
- The Middle East and Africa Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- The Middle East and Africa Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- The Middle East and Africa Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- The Middle East and Africa Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- The Middle East and Africa Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- The Middle East and Africa Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- South Africa
- South Africa Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- South Africa Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- South Africa Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- South Africa Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- South Africa Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- South Africa Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- South Africa Pharmacovigilance Market, by Product Life Cycle
- Saudi Arabia
- Saudi Arabia Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Saudi Arabia Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Saudi Arabia Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Saudi Arabia Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Saudi Arabia Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Saudi Arabia Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Saudi Arabia Pharmacovigilance Market, by Product Life Cycle
- UAE
- UAE Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- UAE Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- UAE Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- UAE Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- UAE Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- UAE Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- UAE Pharmacovigilance Market, by Product Life Cycle
- Kuwait
- Kuwait Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Kuwait Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Kuwait Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Kuwait Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Kuwait Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Kuwait Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Kuwait Pharmacovigilance Market, by Product Life Cycle
- Rest of MEA
- Rest of MEA Pharmacovigilance Market, by Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
- Rest of MEA Pharmacovigilance Market, by Service Provider
- In-house
- Contract Outsourcing
- Rest of MEA Pharmacovigilance Market, by Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
- Rest of MEA Pharmacovigilance Market, by Process flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
- Case Data Management
- Rest of MEA Pharmacovigilance Market, by Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
- Rest of MEA Pharmacovigilance Market, by End Use
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
- Rest of MEA Pharmacovigilance Market, by Product Life Cycle
- The Middle East and Africa Pharmacovigilance Market, by Product Life Cycle
- North America
Pharmacovigilance Market Dynamics
Drivers: Growing Drug Consumption And Drug Development Rates
Drug consumption has increased substantially owing to the rapidly growing prevalence of diseases. The rise in the incidence of chronic diseases, such as cancers, diabetes, and cardiovascular and respiratory disorders, has led to an increase in drug consumption worldwide. According to a WHO report on pharmaceutical consumption, medicines to treat chronic diseases accounted for a more significant proportion of the total volume of drug consumption in nonhospital setups. Furthermore, there has been a substantial rise in the number of drugs made available to healthcare consumers. The rising demand for drugs has significantly increased the need for new drug development via extensive clinical trials. Manufacturers are now focusing on remodeling their drug development processes to cater to patient needs across the globe. The presence of a competitive milieu has led to improved manufacturing operations, pharmacovigilance, clinical data management, streamlined R&D, and medical writing. Manufacturers are rapidly considering outsourcing as a viable cost-curbing tool.
Increasing Incidence Of Adr And Drug Toxicity
ADR imposes a substantial burden on healthcare systems and is one of the prominent causes of morbidity in developed countries. According to the National Center for Biotechnology Information (NCBI), approximately 5% of total hospitalizations in Europe in a year are due to ADR. In February 2022, an article was published in the Journal of Current Medicine Research and Practice titled "Characterization of Seriousness and Outcome of Adverse Drug Reactions in Patients Receiving Cancer Chemotherapy Drugs - A Prospective Observational Study." The article revealed that in the United States, severe Adverse Drug Reactions (ADRs) result in over 100,000 deaths annually, which has been a significant health concern for the past decade. ADRs, like fatal arrhythmia and liver failure, can lead to withdrawal of a drug from the market, as the risks associated with the drug use outweigh its benefits.
Restraints: Shortage Of Skilled Labor
Timely evaluation of drug safety is of paramount importance. Lack of availability of skilled labor, especially in the emerging Asia Pacific region, is expected to restrain market growth over the forecast period. The increase in pharmaceutical and biotechnology companies worldwide has led to a rise in the number of products in the market, which demands efficient PV services. Regulatory policies vary across countries, making it difficult for service providers to meet staffing requirements. Moreover, pharmacovigilance is a continuously evolving process; therefore, researchers need to constantly educate themselves in line with the frequent changes in rules and regulations.
What Does This Report Include?
This section will provide insights into the contents included in this pharmacovigilance market report and help gain clarity on the structure of the report to assist readers in navigating smoothly.
Pharmacovigilance market qualitative analysis
-
Industry overview
-
Industry trends
-
Market drivers and restraints
-
Market size
-
Growth prospects
-
Porter’s analysis
-
PESTEL analysis
-
Key market opportunities prioritized
-
Competitive landscape
-
Company overview
-
Financial performance
-
Product benchmarking
-
Latest strategic developments
-
Pharmacovigilance market quantitative analysis
-
Market size, estimates, and forecast from 2018 to 2030
-
Market estimates and forecast for product segments up to 2030
-
Regional market size and forecast for product segments up to 2030
-
Market estimates and forecast for application segments up to 2030
-
Regional market size and forecast for application segments up to 2030
-
Company financial performance
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationResearch Methodology
A three-pronged approach was followed for deducing the pharmacovigilance market estimates and forecasts. The process has three steps: information procurement, analysis, and validation. The whole process is cyclical, and steps repeat until the estimates are validated. The three steps are explained in detail below:
Information procurement: Information procurement is one of the most extensive and important stages in our research process, and quality data is critical for accurate analysis. We followed a multi-channel data collection process for pharmacovigilance market to gather the most reliable and current information possible.
- We buy access to paid databases such as Hoover’s and Factiva for company financials, industry information, white papers, industry journals, SME journals, and more.
- We tap into Grand View’s proprietary database of data points and insights from active and archived monitoring and reporting.
- We conduct primary research with industry experts through questionnaires and one-on-one phone interviews.
- We pull from reliable secondary sources such as white papers and government statistics, published by organizations like WHO, NGOs, World Bank, etc., Key Opinion Leaders (KoL) publications, company filings, investor documents, and more.
- We purchase and review investor analyst reports, broker reports, academic commentary, government quotes, and wealth management publications for insightful third-party perspectives.
Analysis: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilized different methods of pharmacovigilance market data depending on the type of information we’re trying to uncover in our research.
-
Market Research Efforts: Bottom-up Approach for estimating and forecasting demand size and opportunity, top-down Approach for new product forecasting and penetration, and combined approach of both Bottom-up and Top-down for full coverage analysis.
-
Value-Chain-Based Sizing & Forecasting: Supply-side estimates for understanding potential revenue through competitive benchmarking, forecasting, and penetration modeling.
-
Demand-side estimates for identifying parent and ancillary markets, segment modeling, and heuristic forecasting.
-
Qualitative Functional Deployment (QFD) Modelling for market share assessment.
Market formulation and validation: We mine the data collected to establish baselines for forecasting, identify trends and opportunities, gain insight into consumer demographics and drivers, and so much more. We utilize different methods of data analysis depending on the type of information we’re trying to uncover in our research.
-
Market Formulation: This step involves the finalization of market numbers. This step on an internal level is designed to manage outputs from the Data Analysis step.
-
Data Normalization: The final market estimates and forecasts are then aligned and sent to industry experts, in-panel quality control managers for validation.
-
This step also entails the finalization of the report scope and data representation pattern.
-
Validation: The process entails multiple levels of validation. All these steps run in parallel, and the study is forwarded for publishing only if all three levels render validated results.
Pharmacovigilance Market Categorization:
The pharmacovigilance market was categorized into seven segments, namely product life cycle (Pre-clinical, Phase I, Phase II, Phase III, Phase IV), service provider (In-house, Contract Outsourcing), type (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, EHR Mining), process flow (Case Data Management, Signal Detection, Risk Management System), therapeutic area (Oncology, Neurology, Cardiology, Respiratory Systems), end-use (Pharmaceuticals, Biotechnology Companies, Medical Device Manufacturers), regions (North America, Europe, Asia Pacific, Latin America, Middle East & Africa).
Segment Market Methodology:
The pharmacovigilance market was segmented into product life cycle, service provider, type, process flow, therapeutic area, end-use, and regions. The demand at a segment level was deduced using a funnel method. Concepts like the TAM, SAM, SOM, etc., were put into practice to understand the demand. We at GVR deploy three methods to deduce market estimates and determine forecasts. These methods are explained below:
Market research approaches: Bottom-up
-
Demand estimation of each product across countries/regions summed up to from the total market.
-
Variable analysis for demand forecast.
-
Demand estimation via analyzing paid database, and company financials either via annual reports or paid database.
-
Primary interviews for data revalidation and insight collection.
Market research approaches: Top-down
-
Used extensively for new product forecasting or analyzing penetration levels.
-
Tool used invoice product flow and penetration models Use of regression multi-variant analysis for forecasting Involves extensive use of paid and public databases.
-
Primary interviews and vendor-based primary research for variable impact analysis.
Market research approaches: Combined
- This is the most common method. We apply concepts from both the top-down and bottom-up approaches to arrive at a viable conclusion.
Regional Market Methodology:
The pharmacovigilance market was analyzed at a regional level. The globe was divided into North America, Europe, Asia Pacific, Latin America, Middle East & Africa, keeping in focus variables like consumption patterns, export-import regulations, consumer expectations, etc. These regions were further divided into seventeen countries, namely, the U.S.; Canada; the UK; Germany; France; Italy; Spain; Russia; Japan; China; India; Brazil; Mexico; Argentina, South Africa; Saudi Arabia, UAE.
All three above-mentioned market research methodologies were applied to arrive at regional-level conclusions. The regions were then summed up to form the global market.
Pharmacovigilance market companies & financials:
The pharmacovigilance market was analyzed via companies operating in the sector. Analyzing these companies and cross-referencing them to the demand equation helped us validate our assumptions and conclusions. Key market players analyzed include:
-
ACCENTURE - Accenture is a publicly operated multinational organization involved in management consulting and technological and outsourcing services. Accenture provides solutions for health management, health services, procurement, insurance, marketing, supply chain, and infrastructure services. In addition to the U.S., the company also operates in Europe and Asia. The company's offshore facilities located in India and China are instrumental in providing PV services globally. Accenture, along with Oracle, provides PV consulting services. Accenture offers end-to-end PV services related to medical review, case processing, risk-benefit management, aggregate safety reporting, and operational workflow. The company processes 500,000 ADR and 300 reports annually. For instance, PV consulting, PV technology, and PV outsourcing are three types of services provided by the company.
-
CLINQUEST GROUP B.V. (LINICAL AMERICAS) - Clinquest Services B.V. is Accelovance, Inc. acquired a CRO services provider of Clinquest Group B.V. Clinquest Services B.V. in December 2015. Later, in July 2018, Accelovance, Inc. and Linical merged to create Linical Accelovance Group, a midsized, global CRO offering services across Europe, Asia Pacific, and North America. In May 2020, the parent company's name was changed to Linical Americas. The company offers PV services, post-marketing surveillance, and clinical surveillance services. In addition, the company provides extensive therapeutic expertise in areas ranging from cardiology to reproductive systems.
-
IQVIA - The company was previously known as QuintilesIMS and is now renamed IQVIA. The company provides and serves solutions that allow life sciences companies to maximize opportunities, innovate, and drive human health-related outcomes forward. It focuses on a human data science approach that advances the understanding of human health and allows stakeholders to make insightful, better decisions and discover breakthroughs. The solutions offered by the company include domain expertise, transformative technology, advanced analytics, and unparalleled data.
-
COGNIZANT - Cognizant is involved in business process outsourcing and consulting services. It provides IT system integration and solutions pertaining to finance, healthcare, manufacturing, retail, logistics, communications, media, and entertainment. Cognizant serves the healthcare industry via its two business segments: healthcare and life sciences. It provides consulting services about life sciences, such as drug safety, process consistency, and safety data integration. Cognizant Life Sciences division provides pharmacovigilance services via four platforms: consulting, outsourcing, IT integration, and prebuilt tools.
-
LABORATORY CORPORATION OF AMERICA HOLDINGS - Laboratory Corporation of America Holdings is a clinical laboratory company. Labcorp offers a wide range of procedures and clinical laboratory tests, such as Pap tests, blood chemistry analyzers, substance abuse tests, blood cell counts, and urinalysis. It also offers specialty testing services in cardiovascular diseases, toxicology, diagnostic genetics, and other areas. In addition, the company provides drug development and laboratory testing services. The company serves many hospitals, academic institutions, and universities. In February 2023, LabCorp announced the launch of a new company, Fortrea, a spin-off of its clinical development business. Fortrea will operate as an independent global CRO. It will offer phase I-IV clinical trial services and various commercialization solutions to biotechnology and pharmaceutical organizations.
-
IBM CORPORATION - The company designs, manufactures, and markets software solutions, hardware, hosts, and services. The company operates in over 170 countries. IBM serves its consumers from varied industries, such as automotive, banking, life sciences, retail, chemical and petroleum, and communication. Life science services offered by the company include R&D, supply chain, process, and regulatory compliance. The company also provides healthcare database services.
-
ARISGLOBAL - ArisGlobal is a software solutions provider of cloud-based software solutions to the life sciences industry. ArisGlobal is involved in solutions and products related to various segments, such as Pharmacovigilance (PV) and safety, regulatory affairs, clinical research, and medical information. The company is present globally through registered UK, France, Germany, Ireland, India, and Japan offices. Its cloud-based software platforms are helpful in clinical trials and safety and regulatory information management. SAP-certified solutions provide end-to-end support from data entry to complete automated case submission and the data warehouse system.
-
ICON PLC. - ICON plc is a public limited Contract Research Organization (CRO). It provides services for drug development, such as biometrics, clinical research, data management, interactive technologies, imaging, clinical pharmacology, and consulting and market access solutions. The company is operational in more than 37 countries. ICON Plc has categorized all of its services and solutions under four divisions: laboratory services, early phase services, clinical research services, and DOCS.
-
CAPGEMINI - Capgemini offers technology, consulting, and outsourcing services. The company develops technology projects and provides IT application development, system integration services, and maintenance services. In addition to aerospace and defense, chemicals, automotive, life sciences, healthcare, banking, consumer products, retail, oil & gas, insurance, and telecommunications, the company serves various industries. It provides consulting services in big data and analytics, supply chain management, digital transformation, and sales. The company operates in over 50 countries worldwide.
-
ITCLINICAL - ITClinical is a multinational organization specializing in consulting and information technologies for the health and pharmaceutical industries. The company assists in each step of clinical development and regulatory stages (pre- and postapproval). The primary operational portfolio includes software for clinical trial randomization, PV activities, and clinical trial eCRF. ITClinical provides end-to-end PV services related to medical review, case processing, risk-benefit management, aggregate safety reporting, and operational workflow. The PV system comprises five modules: information, product dictionary, reporting, thesaurus, and management modules.
Value chain-based sizing & forecasting
Supply Side Estimates
-
Company revenue estimation via referring to annual reports, investor presentations, and Hoover’s.
-
Segment revenue determination via variable analysis and penetration modeling.
-
Competitive benchmarking to identify market leaders and their collective revenue shares.
-
Forecasting via analyzing commercialization rates, pipelines, market initiatives, distribution networks, etc.
Demand side estimates
-
Identifying parent markets and ancillary markets
-
Segment penetration analysis to obtain pertinent
-
revenue/volume
-
Heuristic forecasting with the help of subject matter experts
-
Forecasting via variable analysis
Pharmacovigilance Market Report Objectives:
-
Understanding market dynamics (in terms of drivers, restraints, & opportunities) in the countries.
-
Understanding trends & variables in the individual countries & their impact on growth and using analytical tools to provide high-level insights into the market dynamics and the associated growth pattern.
-
Understanding market estimates and forecasts (with the base year as 2022, historic information from 2018 to 2021, and forecast from 2023 to 2030). Regional estimates & forecasts for each category are available and are summed up to form the global market estimates.
Pharmacovigilance Market Report Assumptions:
-
The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue/volume or both. The market for each of the segment outlooks has been provided on region & country basis for the above-mentioned forecast period.
-
The key industry dynamics, major technological trends, and application markets are evaluated to understand their impact on the demand for the forecast period. The growth rates were estimated using correlation, regression, and time-series analysis.
-
We have used the bottom-up approach for market sizing, analyzing key regional markets, dynamics, & trends for various products and end-users. The total market has been estimated by integrating the country markets.
-
All market estimates and forecasts have been validated through primary interviews with the key industry participants.
-
Inflation has not been accounted for to estimate and forecast the market.
-
Numbers may not add up due to rounding off.
-
Europe consists of EU-8, Central & Eastern Europe, along with the Commonwealth of Independent States (CIS).
-
Asia Pacific includes South Asia, East Asia, Southeast Asia, and Oceania (Australia & New Zealand).
-
Latin America includes Central American countries and the South American continent
-
Middle East includes Western Asia (as assigned by the UN Statistics Division) and the African continent.
Primary Research
GVR strives to procure the latest and unique information for reports directly from industry experts, which gives it a competitive edge. Quality is of utmost importance to us, therefore every year we focus on increasing our experts’ panel. Primary interviews are one of the critical steps in identifying recent market trends and scenarios. This process enables us to justify and validate our market estimates and forecasts to our clients. With more than 8,000 reports in our database, we have connected with some key opinion leaders across various domains, including healthcare, technology, consumer goods, and the chemical sector. Our process starts with identifying the right platform for a particular type of report, i.e., emails, LinkedIn, seminars, or telephonic conversation, as every report is unique and requires a differentiated approach.
We send out questionnaires to different experts from various regions/ countries, which is dependent on the following factors:
-
Report/Market scope: If the market study is global, we send questionnaires to industry experts across various regions, including North America, Europe, Asia Pacific, Latin America, and MEA.
-
Market Penetration: If the market is driven by technological advancements, population density, disease prevalence, or other factors, we identify experts and send out questionnaires based on region or country dominance.
The time to start receiving responses from industry experts varies based on how niche or well-penetrated the market is. Our reports include a detailed chapter on the KoL opinion section, which helps our clients understand the perspective of experts already in the market space.
What questions do you have? Get quick response from our industry experts. Request a Free ConsultationShare this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





