- Home
- »
- Pharmaceuticals
- »
-
Systemic Lupus Erythematosus Market, Industry Report 2030GVR Report cover
![Systemic Lupus Erythematosus Market Size, Share & Trends Report]()
Systemic Lupus Erythematosus Market (2024 - 2030) Size, Share & Trends Analysis Report By Drug Class (Biologics, TNF Inhibitors, NSAIDs, Corticosteroids, Antimalarials), By Route of Administration, By Distribution Channel, By Region, And Segment Forecasts
- Report ID: GVR-2-68038-523-6
- Number of Report Pages: 80
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Systemic Lupus Erythematosus Market Summary
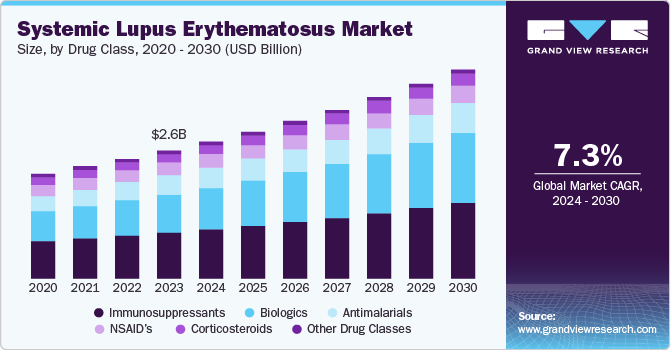
The global systemic lupus erythematosus market size was valued at USD 2.60 billion in 2023 and is projected to reach USD 4.26 billion by 2030, growing at a CAGR of 7.3% from 2024 to 2030. Increasing new cases of autoimmune diseases, growing prevalence of SLE worldwide, awareness regarding significance of early diagnosis, and increasing research and development in treatments for systemic lupus erythematosus (SLE) are some of the key factors influencing market growth.
Key Market Trends & Insights
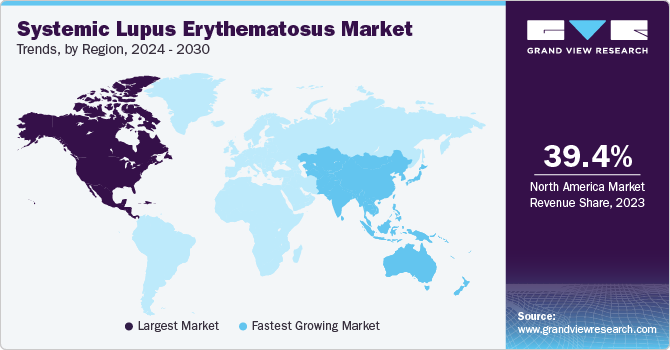
- North America dominated the global systemic lupus erythematosus market and accounted for the largest share of 39.4% in 2023.
- The U.S. systemic lupus erythematosus market dominated the regional market in 2023.
- By drug class, the immunosuppressant segment dominated the global market and accounted for revenue share of 35.1% in 2023.
- By route of administration, the oral route of administration segment accounted for the largest revenue share in 2023.
- By distribution channel, the hospital pharmacy segment dominated the market in 2023.
Market Size & Forecast
- 2023 Market Size: USD 2.60 Billion
- 2030 Projected Market Size: USD 4.26 Billion
- CAGR (2024-2030): 4.2%
- North America: Largest market in 2023
Emergence of innovative therapies related to severe autoimmune diseases, availability of drugs and treatments related to SLE and enhanced healthcare infrastructure in numerous countries has fueled growth of this market in recent years.
Nearly 200,000 individuals in the U.S. are estimated to have SLE. According to the Lupus Foundation of America, SLE is one of the most common lupus, and 7 out of 10 individuals with lupus have SLE. The causes of SLE are still under investigation and not determined, however, it is likely the result of environmental factors and genetics. However, any individual might be diagnosed with SLE; women in the age of 15 to 44 are at higher risk of developing SLE.
Increasing healthcare expenditure, particularly in developed countries, and rising collaborations between pharmaceutical companies and care providers are fostering the development of innovative therapies, thus propelling market growth. Moreover, the presence of regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approving new drugs and therapies more frequently is expected to influence growth of this market during forecast period.
The vast majority of individuals diagnosed with lupus are women, with over 90% of cases occurring in females. Lupus most often strikes during childbearing, typically between the ages of 15 and 45. Similarly, approximately 5 million people worldwide are believed to have lupus, which can cause inflammation and damage to various parts of the body.
Another key growth factor driving the SLE market is the development and approval of new, targeted therapies. Several biopharmaceutical companies are actively pursuing the development of new treatments for systemic lupus erythematosus (SLE). These efforts aim to improve patient outcomes by advancing various SLE research and management aspects. In March 2024, the FDA granted Seattle Children's Hospital an authorization to initiate the first clinical trial in the U.S. utilizing CAR-T cell therapy for children under 18 and old with SLE.
Increasing healthcare expenditure, especially in developed countries, bolsters the adoption of advanced therapies. As governments and private insurers allocate more resources toward treating autoimmune diseases, patients have greater access to new and effective medications. In addition, strategic collaborations and partnerships between pharmaceutical companies, research institutions, and healthcare providers are fostering the development of innovative therapies. Growing awareness regarding the severity of SLE and the need for focused efforts to invent advanced therapies for it is expected to develop growth for this market during the forecast period. In May 2024, Exagen Inc., announced a campaign to raise awareness about lupus during Lupus Awareness Month.
Drug Class Insights
The immunosuppressant segment dominated the global market and accounted for revenue share of 35.1% in 2023. The segment is primarily driven by effectiveness of drug in managing the disease's symptoms. Immunosuppressive drugs are the often prescribed for treating lupus, including methotrexate and azathioprine, which are widely used for their ability to effectively reduce inflammation and modulate the immune response. The growing use, increasing rate of diagnosis resulting from rising awareness and growth in prevalence of SLE are expected to fuel growth of this segment during forecast period.
The biologics segment is anticipated to witness the fastest CAGR from 2024 to 2030. One of the key factors driving the segment growth is the increasing awareness and understanding of the disease's underlying biology. Therapies such as belimumab and anifrolumab have shown high efficacy in treating patients with systemic lupus erythematosus (SLE). The growing demand for personalized medicine and the need for more effective treatments with fewer side effects are driving the adoption of biologics. In addition, the expansion of indications for existing biologics and the emergence of new biologic agents in the pipeline are expected to fuel market growth.
Route of Administration Insights
The oral route of administration segment accounted for the largest revenue share in 2023. Growth of this segment is attributed to its convenience, ease of administration, and high patient compliance. Oral medications such as antimalarial hydroxychloroquine and corticosteroids have been the cornerstone of systemic lupus erythematosus treatment as they offer a convenient and non-invasive treatment option, allowing patients to manage their disease in the comfort of their own homes. In addition, oral medications are often less expensive than biologics and other injectable treatments, making them a more accessible option for patients.
The subcutaneous segment is expected to experience the fastest CAGR of 8.8% over the forecast period. Subcutaneous administration offers improved patient convenience, reduced administration costs, and enhanced treatment adherence.
Distribution Channel Insights
The hospital pharmacy segment dominated the market in 2023. The growth of this segment is attributed to its widespread presence and specialized care offerings. Hospital pharmacies are often equipped with advanced pharmacy information systems and offer various medications, including biologics and immunosuppressant. In addition, hospital pharmacies have trained professionals who provide patient education, counseling, and support as and when needed. Moreover, several health insurance plans cover hospital pharmacy costs, making medications more affordable.
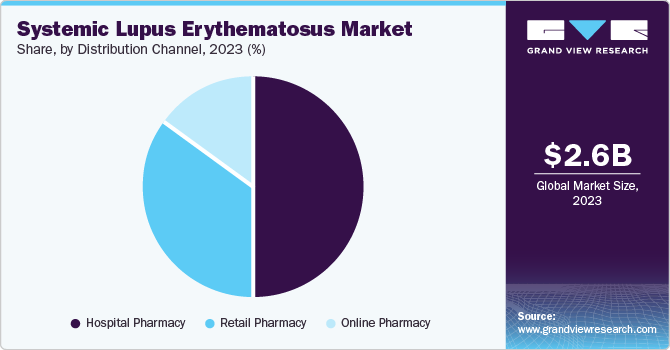
Online pharmacy is anticipated to witness the fastest CAGR during the forecast period. The growing e-commerce sector, the rising demand for convenient and accessible healthcare services, and the adoption of digital technologies are the primary factors that drive the growth of this segment.
Regional Insights
North America dominated the global systemic lupus erythematosus market and accounted for the largest share of 39.4% in 2023. The region's dominance is attributed to the high prevalence of systemic lupus erythematosus, robust healthcare infrastructure, and awareness about autoimmune disease. The region's large pharmaceutical industry, coupled with the presence of key players such as GlaxoSmithKline, Eli Lilly and Company, and AstraZeneca, has driven innovation and investment in the development of new therapies, solidifying North America's position as a leader in the systemic lupus erythematosus market. Furthermore, the well-established regulatory frameworks that streamline the approval and availability of new therapies further contribute to the region's growth. In December 2023, the FDA approved a phase 1 trial of CNTY-101 in patients with moderate to severe systemic lupus erythematosus.
U.S. Systemic Lupus Erythematosus Market Trends
The U.S. systemic lupus erythematosus market dominated the regional market in 2023. The U.S. benefits from an advanced healthcare system with widespread access to specialized medical care, leading to high diagnosis rates and effective management of SLE. Strong pharmaceutical and biotechnology sectors, coupled with a supportive regulatory environment such as the FDA and the Lupus Foundation of America, facilitate the swift approval of new treatments, propelling the region’s growth.
Europe Systemic Lupus Erythematosus Market Trends
Europe held a significant share in the systemic lupus erythematosus market in 2023. The robust presence of well-developed healthcare infrastructure, increasing prevalence of SLE, and wide healthcare coverage are the key factors driving the region’s growth. The region is home to numerous research institutions and pharmaceutical companies that contribute to developing and available innovative SLE treatments.
Asia Pacific Systemic Lupus Erythematosus Market Trends
Asia Pacific systemic lupus erythematosus market is anticipated to witness the fastest CAGR of 8.0% in 2023. Increasing SLE disease, awareness, and healthcare expenditure are the key factors expected to drive the region’s growth. SLE is more common among Asian populations, and China and India, being the most populous countries, naturally have a higher number of patients diagnosed with this autoimmune condition.
Key Systemic Lupus Erythematosus Company Insights
Some of the key companies in the systemic lupus erythematosus market include Novartis AG, GSK plc, F. Hoffmann-La Roche Ltd, Sanofi, Bristol-Myers Squibb Company, and others. To address the growing competition, the major market participants are adopting strategies such as enhanced focus on research and development, innovation-based new product launches, collaborations, and acquisitions of other organizations operating in similar businesses.
-
GSK plc, a global pharmaceuticals and biotechnology company, offers a diverse range of products across three different categories, including vaccines, specialty, and general medicines. One of its key offerings in systemic lupus erythematosus (SLE) treatment is Benlysta (belimumab), first approved in 2011, a significant medication for adults and pediatric patients with SLE.
Key Systemic Lupus Erythematosus Companies:
The following are the leading companies in the systemic lupus erythematosus market. These companies collectively hold the largest market share and dictate industry trends.
- Novartis AG
- GSK plc.
- F. Hoffmann-La Roche Ltd
- Pfizer Inc.
- Sanofi
- Lycera (Celgene)
- Bristol-Myers Squibb Company
- ImmuPharma PLC
- Merck KgaA
- AstraZeneca
- UCB S.A.
Systemic Lupus Erythematosus Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 2.79 billion
Revenue Forecast in 2030
USD 4.26 billion
Growth rate
CAGR of 7.3% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million and CAGR from 2024 to 2030
Report Coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments Covered
Drug class, route of administration, distribution channel, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, Germany, UK, France, Italy, Spain, China, Japan, Australia, South Korea, India, Brazil, Argentina, South Africa, Saudi Arabia, UAE
Key companies profiled
Novartis AG; GSK plc.; F. Hoffmann-La Roche Ltd; Pfizer Inc.; Sanofi; Lycera (Celgene); Bristol-Myers Squibb Company; ImmuPharma PLC; Merck KgaA; AstraZeneca; UCB S.A.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Systemic Lupus Erythematosus Market Report Segmentation
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global systemic lupus erythematosus market report based on drug class, route of administration, distribution channel, and region:
-
Drug Class Outlook (Revenue, USD Million, 2018 - 2030)
-
Biologics
-
Saphnelo (Anifrolumab)
-
Benlysta (Belimumab)
-
Phase 3 Pipeline Products
-
-
TNF Inhibitors
-
NSAID’s
-
Corticosteroids
-
Antimalarials
-
Immunosuppressants
-
Other Drug Classes
-
-
Route of Administration Outlook (Revenue, USD Million, 2018 - 2030)
-
Oral
-
Intravenous
-
Subcutaneous
-
Other Route of Administration
-
-
Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital Pharmacy
-
Retail Pharmacy
-
Online Pharmacy
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










