- Home
- »
- Pharmaceuticals
- »
-
North America Active Pharmaceutical Ingredients Market, Industry Report, 2030GVR Report cover
![North America Active Pharmaceutical Ingredients Market Size, Share & Trends Report]()
North America Active Pharmaceutical Ingredients Market Size, Share & Trends Analysis Report By Type Of Synthesis (Biotech, Synthetic), By Type Of Manufacturer (Captive, Mesrchant), By Type, By Application, By Type Of Drug, By Country, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-4-68040-221-1
- Number of Report Pages: 80
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2024 - 2030
- Industry: Healthcare
North America API Market Size & Trends
The North America active pharmaceutical ingredients market size was estimated at USD 90.88 billion in 2023 and is expected to grow at a CAGR of 4.7% from 2024 to 2030. Increasing prevalence of infectious diseases and hospital-acquired infections coupled with growing prevalence of lifestyle induced conditions is one of the high impact rendering drivers of active pharmaceutical ingredients (API) market.
Lifestyle significantly affects the mental and physical health of human beings. The increasing adoption of unhealthy behaviors such as smoking, physical inactivity, and an unhealthy diet, along with the rising cases of hospital-acquired infections, are factors expected to propel market growth. According to the Canadian Patient Safety Institute, approximately 8,000 patients in Canada die due to hospital-acquired infections, and about 220,000 patients are infected with such diseases. Moreover, the prevalence of stroke is rapidly increasing in the region. The Centers for Disease Control and Prevention (CDC) reports that about 795,000 people in the U.S. suffer from stroke yearly, with 87% being ischemic stroke. Hence, the demand for drugs such as thrombolytic agents for treatment is expected to increase significantly.
In the wake of COVID-19 pandemic and broader reshoring of API production, there is now a stronger emphasis on bringing clinical product supply security back to the U.S., where clinical studies are conducted. Increasing government initiatives to support API production for securing a robust supply of raw materials for pharmaceutical production is expected to drive market growth. The government has devised regulatory changes and announced policies to aid in API manufacturing facility setup. For instance, in June 2021, policy changes were recommended by the Biden Administration to reduce dependence on imports, boosting API production in the U.S. Moreover, in September 2022, President Biden launched the National Biomanufacturing & Biotechnology initiative to provide new investments and resources in the country.
Market Concentration & Characteristics
The market growth stage is medium and pace of the market growth is accelerating. Precision medicine has significantly impacted the API industry, fueling the demand for specialized APIs and novel approaches for drug testing & development. For instance, cancer treatments for particular genetic mutations are under development, and therapies for rare genetic disorders are being developed, for specific gene defects. Hence, this increased the demand for specialized APIs, which can be manufactured and processed at a wider scale.
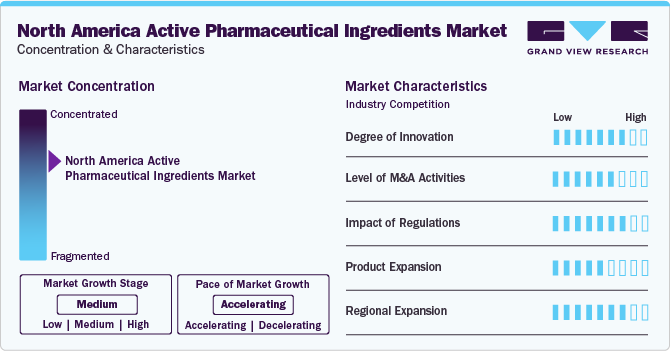
North America active pharmaceutical ingredients (API) market is witnessing an increasing number of merger & acquisition (M&A) activities that are being undertaken by the prominent companies. Several North American medical imaging companies are adopting these strategies to upgrade their portfolio in the region. For instance, in June 2021, Sun Pharmaceutical Industries Ltd. signed a supply and license agreement with Cassiopea SpA to distribute Winlevi in the U.S. and Canada.
Stringent regulatory oversight by agencies like the FDA and EMA has compelled API manufacturers to prioritize quality & safety, resulting in compliance with GMPs and stringent quality standards. Several key players are undergoing legal scrutiny because of non-adherence to standards. For instance, in August 2019, the U.S. FDA issued 75 warning letters to API production facilities. In November 2022, AbbVie settled a pay-for-delay to resolve Nameda lawsuit for USD 54 million. Such stringent regulations are anticipated to act as a restraining factor for market growth.
Companies are devising innovative product development strategies and focusing on expanding their regional business footprints, which is driving the product demand. For instance, in June 2023, AGEPHA Pharma USA, LLC, received FDA approval for LODOCO, a groundbreaking anti-inflammatory atheroprotective cardiovascular treatment. This drug has been shown to reduce the risk of heart attacks, strokes, and other cardiovascular events in adult patients with established atherosclerotic disease or multiple cardiovascular risk factors. The FDA’s prioritized review underscores the significance of this breakthrough in cardiovascular medicine.
Prominent active pharmaceutical ingredients companies in North America, including AbbVie Inc., Pfizer Inc., and Viatris Inc. among others have a significant presence in the region. However, a number of companies in Europe and Asia Pacific region are focusing to initiate their operations in North America. For instance, in May 2022, Piramal Pharma established a new API plant in Aurora, Ontario, with a manufacturing site of more than 10,000 square feet.
Type Of Synthesis Insights
The synthetic segment held the largest revenue share of 72% in 2023. Major driver for synthetic API market is high demand for generic drugs. APIs used to develop generic drugs contribute to high revenue for synthetic and chemical API manufacturing companies. This is creating a wide opportunity for CDMOs. An increase in outsourcing trend for improving profitability by reducing the cost of production is creating new growth avenues for the market.
Biotech API is expected to witness fastest growth during the forecast period. Growth of biotech API segment can be attributed to rising investments in biopharmaceutical and biotechnology sectors. This allows innovation of new molecules that aid in treating diseases such as cancer. Key players are highly focused on biotech APIs owing to high revenue generation and profitability. Some of the technological advancements in biotech API manufacturing include single-use bioreactors, which are made of plastic and have been sterilized and sealed with gamma radiation.
Type Of Manufacturer Insights
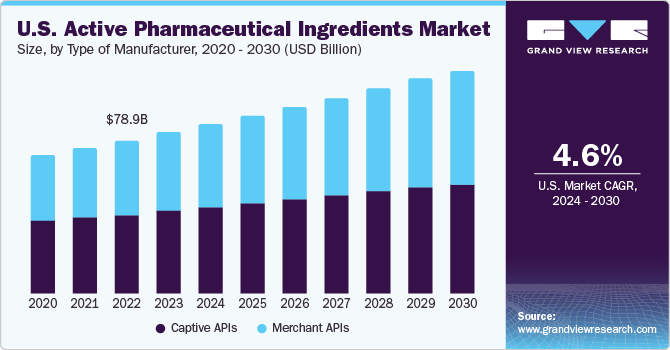
Captive APIs segment held the largest market share of 51.2% in 2023. Numerous companies are investing in solving challenges and developing new chemical ways for in-house production of APIs. This aids in reducing costs and the risk of contamination. Protein synthesis and artificial intelligence are expected to accelerate development with greater control over the process. Furthermore, recent initiatives and developments by key players indicate a strong preference for in-house manufacturing over outsourcing. For instance, in September 2021, AstraZeneca announced an investment of USD 360 million in its API manufacturing facility in Ireland to commercialize novel products. Such initiatives undertaken by key players are anticipated to boost segment growth.
Merchant APIs segment is expected to experience the fastest growth over the forecast period. Contract manufacturing and outsourcing of API molecule development are growing trends in the pharmaceutical sector. As captive production of APIs is expensive, companies have started opting for outsourcing to minimize expenses. Merchant APIs eliminate the need for investing in expensive equipment and sophisticated infrastructure. Post-pandemic, key companies are expanding their capacities to enhance their market presence. For instance, in May 2023, MilliporeSigma announced the expansion of its U.S.-based facility, with an investment of USD 69 million, doubling its manufacturing capacity for Highly Potent Active Pharmaceutical Ingredient (HPAPI). The facility is dedicated to the development and commercial manufacturing of Antibody Drug Conjugates (ADCs).
Type Insights
Innovative APIs segment dominated the overall API market with a revenue share of 67.5% in 2023. Furthermore, it the segment is also anticipated to grow at the fastest CAGR. Furthermore, the segment is also anticipated to grow at the fastest CAGR. The growth is attributed to an increase in funding and favorable regulations for R&D facilities. Many novel innovative products are now in the pipeline due to extensive research in this field and are expected to be launched in the near future. Furthermore, increasing support from regulatory agencies for the approval of new drugs is projected to facilitate market growth, which can be attributed to an increase in the focus of the government on healthcare and pharmaceuticals due to COVID-19.
Application Insights
Cardiology segment dominated the API market with a revenue share of 21.4% in 2023, attributable to the rising prevalence of cardiovascular diseases. Cardiovascular disease is one of the world's most serious public health problems, prompting extensive research into APIs in the field. Government initiatives, such as the National Cholesterol Education Program, are aimed at raising awareness related to lipid and cholesterol-related diseases. These programs also support medication-based awareness. High prevalence and increasing awareness about cardiovascular diseases are anticipated to boost segment growth over the forecast period, driving the demand for APIs for cardiology drugs.
Oncology segment is anticipated to grow at the fastest rate over the forecast period. Increasing prevalence of cancer is a key factor driving this market. Collaboration among pharmaceutical companies, research institutions, and regulatory entities remains pivotal in expediting drug development, ensuring patient safety, and fostering innovation. For instance, in March 2023, Pfizer Inc. and Seagen Inc. confirmed a definitive merger agreement. Through this strategic deal, Pfizer planned to acquire Seagen, a renowned biotech firm specializing in revolutionary cancer treatments. The agreement includes a cash transaction of USD 229 per Seagen share, resulting in an enterprise value of USD 43 billion.
Type Of Drug Insights
Prescription dominated the market with a revenue share of 81% in 2023. The uptake of prescription drugs is largely dependent on physicians’ prescriptions. Use of prescription drugs, such as Proton Pump Inhibitors (PPI), in the management of general conditions, heartburn, has plateaued owing to several adverse effects. However, Histamine-2 Receptor Antagonist (H2RA) prescription rate has been impacted. Prescription drugs dominated in oncology segment as cancer is primarily treated using chemotherapy, targeted therapy, immunotherapy, and hormonal therapy. The use of biology is also increasing.Due to the increased efficacy of novel targeted therapies, the number of prescriptions for targeted therapies is rapidly increasing. Furthermore, major players are launching novel targeted therapies.
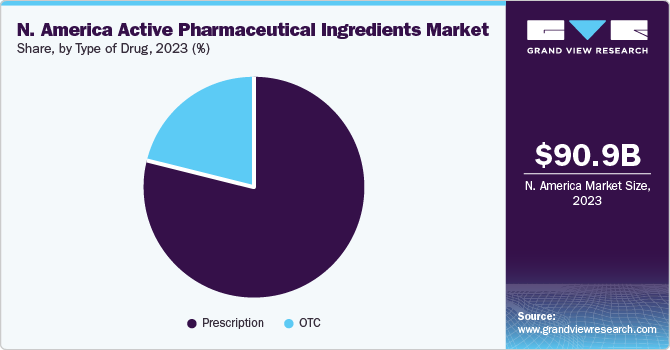
OTC segment is expected to depict the fastest growth over the forecast period. OTC products are easily accessible to the population and is frequently impacted by changes in consumer behavior. Consumer preference is shifting from use of antacids for heartburn to ensuring gut health by taking probiotics. This paradigm shift is creating greater opportunities for preventive products, such as health supplements, nutraceuticals, and probiotics, while slashing the growth of existing products.
Country Insights
North America accrued the largest revenue share of 38.3% in 2023 owing to rising prevalence of cardiovascular, genetic, and other chronic diseases aided with growing research in field of drug development. The region shows high value manufacturing areas, including complex & high potent APIs, gene therapies & biologicals, which is expected to provide relative growth. Moreover, there is significant expansion of innovators and CDMOs seen in the region, which is creating an added advantage for manufacturing and commercializing APIs.
U.S. Active Pharmaceutical Ingredients Market Trends
U.S. led the market with a share of 91.8% in 2023. Presence of key players such as AbbVie Inc.; Curia; Pfizer Inc. (Pfizer Center One); Viatris Inc.; and Fresenius Kabi AG is positively influencing the market growth. For instance, in February 2022, Viatris received FDA approval for Generic Restasis—a cyclosporine ophthalmic emulsion for the treatment of dry eye disease.
Canada Active Pharmaceutical Ingredients Market Trends
Canada is projected to depict the fastest growth rate over the forecast period. The growing interest of key players in entering the Canadian market is opportunistic for the market growth. For instance, in March 2022, Viatris Inc., in collaboration with Biocon, launched oncology biosimilar Abevmy in Canada. Moreover, in July 2021, MediPharm Labs received a license from Health Canada for manufacturing and sale of cannabis API in Canada. In January 2021, 5N Plus, Inc. announced its expansion into the API segment to include new class of antibiofilm and antibiotic drugs.
Key North America Active Pharmaceutical Ingredients Company Insights
AbbVie Inc., Pfizer Inc., and Viatris Inc. are some of the key companies in the market. Presence of pipeline products in the API market is expected to launch in the coming years and is anticipated to drive market growth. Further, increasing outsourcing activities due to high manufacturing costs and stringent regulations on the production of APIs are expected to maintain the competitive rivalry at a high level during the forecast period.
-
In October 2023, Cambrex announced the completion of its USD 38 million small molecule API manufacturing facility. This investment doubled the size of the company’s manufacturing facility and enhanced its ability to acquire more customers to meet their evolving needs.
-
In August 2023, BARDA and Regeneron entered into an agreement wherein the former will support the latter in developing an antibody therapy to prevent SARS-CoV-2. This contract is worth USD 326 million and is anticipated to drive the market with production of novel vaccines in the coming years.
-
In April 2023, Eli Lilly announced an investment of USD 1.6 billion in U.S.-based LEAP Innovation Park. This brings the total investment to USD 3.7 billion to manufacture complex APIs for products such as genetic medicine.
-
In April 2022, Geocann entered into a strategic partnership with Averix Bio for supplying API phytocannabinoid ingredients.
Key North America Active Pharmaceutical Ingredients Companies:
- Dr. Reddy’s Laboratories Ltd.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Cipla Inc.
- AbbVie Inc.
- Aurobindo Pharma
- Sandoz International GmbH (Novartis AG)
- Viatris Inc.
- Fresenius Kabi AG
- STADA Arzneimittel AG
- Lonza
- Curia
- Pfizer Inc.
- Bristol-Myers Squibb Company
- Merck KGaA
- Catalent, Inc.
North America Active Pharmaceutical Ingredients Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 95.63 billion
Revenue forecast in 2030
USD 125.84 billion
Growth rate
CAGR of 4.7% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion, and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type of synthesis, type of manufacturer, type, application, type of drug, country
Regional scope
North America
Country scope
U.S.; Canada
Key companies profiled
Dr. Reddy’s Laboratories Ltd.; Sun Pharmaceutical Industries Ltd.; Teva Pharmaceutical Industries Ltd.; Cipla Inc.; AbbVie Inc.; Aurobindo Pharma; Sandoz International GmbH (Novartis AG); Viatris Inc.; Fresenius Kabi AG; STADA Arzneimittel AG; Lonza; Curia; Pfizer Inc.; Bristol-Myers Squibb Company; Merck KGaA; Catalent, Inc.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
North America API Market Report Segmentation
This report forecasts revenue growth in the North America market and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the North America active pharmaceutical ingredients market report based on type of synthesis, type of manufacturer, type, application, type of drug, and country:
-
Type of Synthesis Outlook (Revenue, USD Billion, 2018 - 2030)
-
Biotech
-
Biotech APIs Market, by Type (Revenue, USD Billion, 2018 - 2030)
-
Generic APIs
-
Innovative APIs
-
-
Biotech APIs Market, by Product (Revenue, USD Billion, 2018 - 2030)
-
Monoclonal Antibodies
-
Hormones
-
Cytokines
-
Recombinant Proteins
-
Therapeutic Enzymes
-
Vaccines
-
Blood Factors
-
-
-
Synthetic
-
Synthetic APIs Market, by Type (Revenue, USD Billion, 2018 - 2030)
-
Generic APIs
-
Innovative APIs
-
-
-
-
Type of Manufacturer Outlook (Revenue, USD Billion, 2018 - 2030)
-
Captive APIs
-
Merchant APIs
-
-
Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Generic APIs
-
Innovative APIs
-
-
Application Outlook (Revenue, USD Billion, 2018 - 2030)
-
Cardiovascular Diseases
-
Oncology
-
CNS and Neurology
-
Orthopedic
-
Endocrinology
-
Pulmonology
-
Gastroenterology
-
Nephrology
-
Ophthalmology
-
Others
-
-
Type of Drug Outlook (Revenue, USD Billion, 2018 - 2030)
-
Prescription
-
OTC
-
-
Country Outlook (Revenue, USD Billion, 2018 - 2030)
-
U.S.
-
Canada
-
Frequently Asked Questions About This Report
b. The North America active pharmaceutical ingredients market size was estimated at USD 90.88 billion in 2023 and is expected to reach USD 95.63 billion in 2024.
b. The North America active pharmaceutical ingredients market is expected to grow at a compound annual growth rate of 4.7% from 2024 to 2030 to reach USD 125.84 billion by 2030.
b. Cardiology segment dominated the North America API market with a revenue share of 21.4% in 2023, attributable to the rising prevalence of cardiovascular diseases. Cardiovascular disease is one of the world's most serious public health problems, prompting extensive research into APIs in the field.
b. Key players in the North America API market are Dr. Reddy’s Laboratories Ltd.; Sun Pharmaceutical Industries Ltd.; Teva Pharmaceutical Industries Ltd.; Cipla Inc.; AbbVie Inc.; Aurobindo Pharma; Sandoz International GmbH (Novartis AG); Viatris Inc.; Fresenius Kabi AG; STADA Arzneimittel AG; Lonza; Curia; Pfizer Inc.; Bristol-Myers Squibb Company; Merck KGaA; Catalent, Inc.
b. The active pharmaceutical ingredients market growth can be attributed to increasing prevalence of infectious diseases and hospital-acquired infections coupled with growing prevalence of lifestyle induced conditions and trend of outsourcing API production.
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





