- Home
- »
- Clinical Diagnostics
- »
-
U.S. Breast Cancer Diagnostics Market, Industry Report 2033GVR Report cover
![U.S. Breast Cancer Diagnostics Market Size, Share & Trends Report]()
U.S. Breast Cancer Diagnostics Market (2025 - 2033) Size, Share & Trends Analysis Report By Product (Platform-based, Instrument-based), By Type (Imaging, Biopsy, Genomic Tests, Blood Tests), By Application, By End-use, And Segment Forecasts
- Report ID: GVR-4-68040-726-1
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2021 - 2023
- Forecast Period: 2025 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
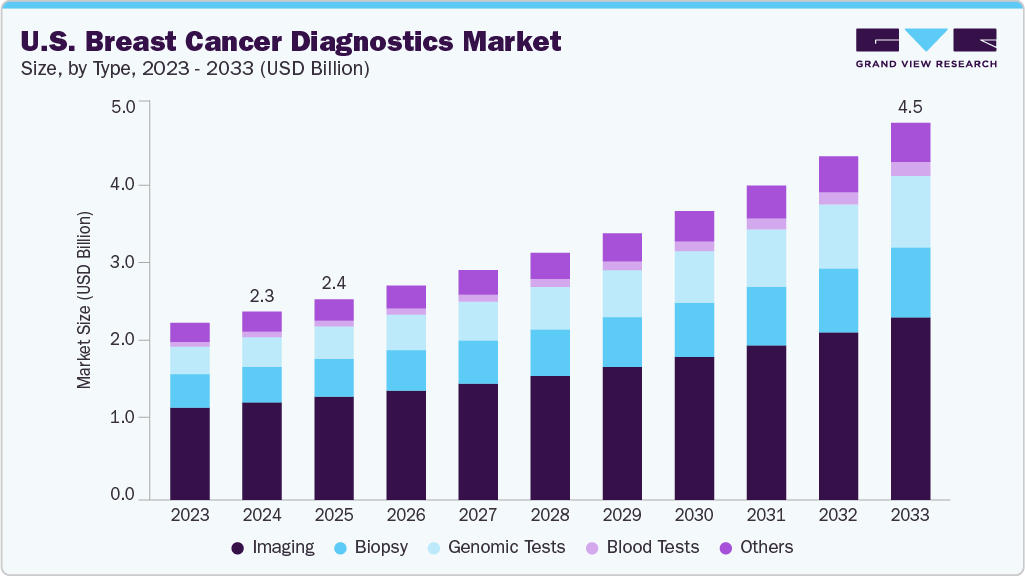
The U.S. breast cancer diagnostics market size was estimated at USD 2.26 billion in 2024 and is projected to reach USD 4.53 billion by 2033, registering a CAGR of 8.21% from 2025 to 2033. The growth can be attributed to the increasing prevalence of breast cancer and rising government initiatives to increase the screening & diagnostic rate. For instance, according to the American Cancer Society, Breast cancer remains the most prevalent cancer among women in the U.S., excluding skin cancers, accounting for nearly 30% of all new female cancer cases annually. In 2025, an estimated 316,950 new cases of invasive breast cancer and 59,080 cases of ductal carcinoma in situ (DCIS) were diagnosed. Tragically, 42,170 women are expected to die from the disease. The increasing incidence of breast cancer fuels demand for advanced diagnostic technologies such as mammography, genetic testing, and AI-powered imaging. Rising awareness, government initiatives, and expanding insurance coverage further stimulate the adoption of screening programs and innovative diagnostics across the U.S.
The market in the U.S. is supported by a high disease prevalence, strong policy initiatives, and rapid technological innovation. Breast cancer remains the most common cancer among U.S. women, with the American Cancer Society projecting over 316,950 invasive cases in 2025. Rising incidence in younger demographics, coupled with growing awareness about the importance of early detection, is fueling demand for advanced diagnostic tools. The U.S. government and research institutions are actively promoting innovation through grants and funding. For example, in January 2024, Weill Cornell Medicine received a $2.4 million Department of Defense grant to validate the Syantra DX liquid biopsy test, an AI-driven blood-based screening solution that could significantly improve access for women with dense breast tissue, underserved populations, and high-risk groups. The availability of non-invasive, cost-effective tests is expected to improve early detection, reduce aggressive treatments, and shift market demand away from traditional imaging toward liquid biopsy technologies.
The launch of innovative intraoperative diagnostic solutions is also transforming breast cancer care. In January 2025, Lumicell introduced the LumiSystem, the first FDA-approved real-time fluorescence-guided imaging tool for lumpectomy procedures. By integrating the LUMISIGHT optical imaging agent with the Direct Visualization System (DVS), surgeons are able to identify and remove cancerous tissue intraoperatively, thereby minimizing the need for repeat surgeries and improving patient outcomes. Such advancements not only enhance surgical precision but also reduce long-term healthcare costs. Key industry players, including Roche, Thermo Fisher Scientific, QIAGEN, BD, and Danaher, are investing heavily in regulatory approvals and partnerships to expand their market presence. For instance, Roche’s PATHWAY HER2 (4B5) test has been granted successive FDA approvals, first for identifying HER2-low and more recently HER2-ultralow metastatic breast cancer patients eligible for ENHERTU therapy, further strengthening the role of precision diagnostics in treatment selection. Similarly, PreludeDx’s DCISionRT test received FDA Breakthrough Device designation in 2025, enabling physicians to personalize treatment decisions for ductal carcinoma in situ (DCIS) patients and avoid unnecessary interventions.
Biopsy remains a fundamental diagnostic method in the U.S., with more than 1 million procedures conducted annually, of which around 20% confirm malignancy. Needle-based biopsies-comprising fine needle aspiration, core needle, and vacuum-assisted biopsies-represent over 90% of cases due to their minimally invasive nature and higher patient compliance compared to surgical biopsies. The segment is witnessing notable innovation, with Mammotome introducing the AutoCore Single Insertion Core Biopsy System in November 2024, which improves efficiency through real-time visualization, single insertion sampling, and touchless specimen transfer. At the same time, liquid biopsy is rapidly gaining ground, with the FDA approval of FoundationOne Liquid CDx in October 2024 as a companion diagnostic for Itovebi, targeting HR-positive, HER2-negative patients with PIK3CA mutations. These advancements highlight a broader trend toward minimally invasive and precision-guided testing solutions in the U.S. diagnostics market.
Alongside biopsy advancements, the U.S. is experiencing significant adoption of genomic profiling and AI-driven diagnostic tools. In August 2024, Illumina expanded the genomic testing landscape with its NovaSeq X Series, including the high-throughput TruSight Oncology 500 HT and the latest liquid biopsy assay, ctDNA v2, enabling larger-scale and faster genomic profiling. Such developments are pivotal in driving the adoption of comprehensive genomic profiling (CGP) across breast cancer care. Complementing this, GE HealthCare launched the MyBreastAI Suite in 2023, which integrates AI-driven breast density assessment and radiology workflow enhancements, supporting radiologists in early detection and improving operational efficiency. Together, these solutions illustrate the market’s shift toward digitally enabled precision diagnostics, which offer greater accuracy, speed, and scalability while helping clinicians optimize patient care pathways.
The U.S. regulatory and reimbursement framework plays a crucial role in shaping market adoption. The FDA ensures diagnostic test safety and effectiveness, while the Centers for Medicare & Medicaid Services (CMS) oversees laboratory compliance under the Clinical Laboratory Improvement Amendments (CLIA). Regulations such as the Mammography Quality Standards Act (MQSA) mandate accreditation, annual inspections, and standardized reporting for mammography facilities, safeguarding quality and reliability. A new rule effective September 2024 requires mammography providers to include breast density assessments in patient reports, driving the need for supplemental diagnostic tools and informed decision-making. On the reimbursement front, both Medicare and private insurers cover genetic testing, including BRCA1 and BRCA2, particularly for individuals with family histories of breast cancer. Reimbursement through the Clinical Laboratory Fee Schedule (CLFS) continues to ensure broader patient access, supporting higher test volumes nationwide. With strong regulatory oversight, favorable reimbursement, and a pipeline of technological innovations, the U.S. breast cancer diagnostics market is well-positioned for sustained growth, advancing toward more accessible, precise, and patient-centric solutions.
Market Concentration & Characteristics
The industry demonstrates a high degree of innovation, driven by rapid advancements in liquid biopsy, genomic profiling, fluorescence-guided imaging, and AI-enabled solutions. Emerging tools such as Syantra DX, a blood-based AI diagnostic, and LumiSystem, an intraoperative imaging system, highlight the shift toward non-invasive and precision-guided care. Companies are also focusing on comprehensive genomic profiling (CGP) and integrating AI to improve accuracy, speed, and cost-effectiveness. These innovations are reshaping diagnostic pathways, enabling earlier detection, reducing repeat surgeries, and expanding access for underserved populations, ultimately strengthening patient outcomes.
Mergers and acquisitions (M&A) activity in the industry is robust, with major players such as Roche, Danaher, Thermo Fisher Scientific, and QIAGEN pursuing acquisitions to strengthen their diagnostic portfolios. These transactions often aim to expand genomic capabilities, enhance AI-driven tools, or integrate novel liquid biopsy platforms. Acquisitions also provide access to FDA-approved companion diagnostics and precision medicine solutions, accelerating market penetration. The consolidation trend reflects the industry’s strategy to combine complementary technologies, scale operations, and maintain competitive leadership in an evolving, innovation-driven diagnostics ecosystem.
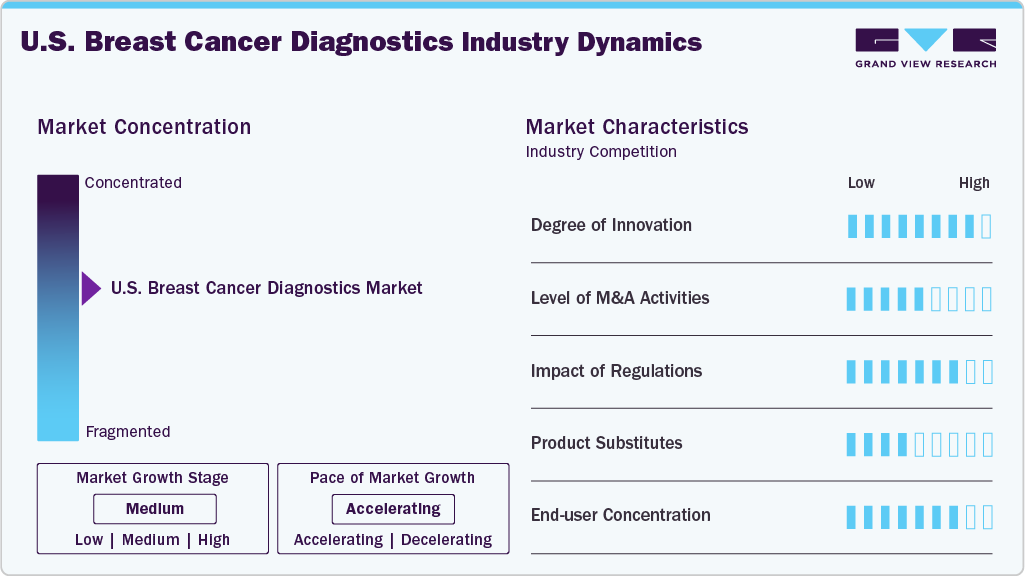
Regulation plays a defining role in the industry, ensuring patient safety, reliability, and quality across diagnostic platforms. The FDA oversees product safety, effectiveness, and approvals, while the CMS regulates laboratory compliance through CLIA standards. The Mammography Quality Standards Act (MQSA) mandates accreditation and inspection of mammography facilities, ensuring consistent national quality. Recently, regulations requiring the inclusion of breast density assessments in mammogram reports (effective September 2024) emphasize transparency and patient empowerment. These frameworks, while rigorous, also accelerate the adoption of advanced tools by establishing clear safety benchmarks and boosting clinical trust.
Product expansion is a key growth driver in the U.S. market, as companies diversify portfolios to address evolving clinical needs. Launches such as Lumicell’s LumiSystem, Mammotome’s AutoCore Biopsy System, and Illumina’s TruSight Oncology 500 HT reflect a push toward real-time imaging, non-invasive biopsy, and large-scale genomic profiling. Roche continues to expand with successive approvals of the PATHWAY HER2 (4B5) test, targeting HER2-low and HER2-ultralow patients. These new product introductions not only enhance clinical outcomes but also broaden market opportunities, reinforcing the trend toward precision, speed, and patient-centric diagnostic innovations.
Regional expansion within the U.S. is driven by the need to increase access in underserved and rural populations. Innovations like liquid biopsy tests (e.g., Syantra DX) and AI-driven platforms enable wider deployment beyond major urban hospitals, reducing dependence on centralized imaging centers. Leading companies are also expanding collaborations with community healthcare providers and regional cancer centers to accelerate adoption. Furthermore, favorable reimbursement under Medicare and private insurers encourages broader test accessibility nationwide. This regional scaling improves diagnostic reach, reduces disparities in early detection, and supports overall market penetration.
Type Insights
The imaging segment dominated the market and held the largest revenue share of 51.77% in 2024. The industry witnessed significant growth in 2024 due to the widespread adoption of imaging techniques like mammography, ultrasound, and MRI. These modalities have become the primary tools for breast cancer diagnosis, while advanced technologies such as MBI, CT, 3D breast tomosynthesis, and PET show great potential for transforming breast imaging capabilities.
In September 2024, the CDC reported breast cancer to be the second most prevalent type of cancer and the second leading cause of death among women in the United States. On October 1, 2024, Barco, a global producer of breast imaging displays, unveiled the Coronis OneLook display solution. This advanced display has been optimized for interpreting 3D cine examinations from CT, ultrasound, and breast MRI. Equipped with Barco's patented RapidFrame technology, it ensures crisp moving images without blur. Additionally, Coronis OneLook offers customizable on-screen touch buttons, enhancing workflow efficiency for radiologists. In addition to the hardware, Barco launched the ConnectCare service, enhancing diagnostic display management for healthcare organizations for weekly monitoring and automated compliance reporting.
The blood tests segment is anticipated to register a lucrative CAGR in the coming years, driven by extensive research studies conducted by research organizations and major players. The high effectiveness of liquid biopsy tests contributes to the growth in the number of blood tests. In March 2025, BCAL Diagnostics Limited, a biotechnology company operating in both Australia and the US, officially launched BREASTEST plus. The company marks the challenge of detecting lesions in high breast density. This innovative blood test is designed to complement standard imaging methods in breast cancer screening and diagnostics. BREASTEST plus aims to enhance early breast cancer detection by providing a non-invasive alternative to traditional mammograms. In clinical studies, it demonstrated a sensitivity of 90% and a specificity of 85.5%, outperforming existing screening methods. In March 2025, Personalis Inc., along with The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, highlighted the importance of ultra-sensitive ctDNA for early-stage breast cancer recurrence detection using NeXT Personal. Personalis' NeXT Personal assay is an ultra-sensitive, personalized liquid biopsy test designed to detect minimal amounts of circulating tumor DNA (ctDNA) from the blood of cancer patients and survivors. This information enables the creation of a customized blood test capable of detecting these specific variants with exceptional sensitivity, down to approximately one part per million (PPM) of ctDNA.
Product Insights
The instrument-based products segment dominated the market and held the largest revenue share of 70.64% in 2024. Instrument-based products offer high precision and accuracy in detecting and diagnosing the disease. These instruments are equipped with advanced imaging technologies and molecular analysis tools that provide detailed and reliable information about the presence, location, and characteristics of tumors. This accuracy is crucial for guiding treatment decisions and ensuring optimal patient outcomes. Instrument-based products play a pivotal role in breast cancer screening and early detection. Mammography machines, for example, are widely used for routine screening due to their ability to detect tumors at an early stage when they are most treatable.
On the other hand, the platform-based products segment is expected to witness the highest CAGR growth rate during the forecast period. Platform-based products offer comprehensive solutions that encompass multiple diagnostic modalities and technologies within a single integrated system. Furthermore, Platform-based products streamline workflows by consolidating diagnostic procedures, leading to time and cost savings for healthcare providers. Additionally, the availability of approved products such as Ion GeneStudio S5 next-generation sequencing systems, Ion AmpliSeq HD panels, and GeneReader NGS system for targeted NGS and QIAseq Panel assays is accountable for segment growth.
Application Insights
The diagnostic & predictive segment dominated the market and held the largest revenue share of 43.29% in 2024. Diagnostic applications play a crucial role in identifying the presence of breast cancer at an early stage. Early detection enables timely intervention, leading to improved treatment outcomes and reduced mortality rates. Diagnostic methods such as mammography, ultrasound, and MRI help detect abnormalities in breast tissue, guiding healthcare providers in making accurate diagnoses. Recent advancements in this field include the development of a liquid biopsy test, showcased in a study published in January 2023, which utilizes circulating tumor DNA to detect early-stage breast cancer with high accuracy.
On the other hand, the prognostic segment is expected to witness the highest CAGR growth rate during the forecast period. The market for prognostic tests in breast cancer is highly competitive, with numerous players vying for the same patient base. The increasing importance of prognostic tests in matching patients with appropriate therapies has significantly improved survival rates. Companion diagnostics, such as BRAC Analysis CDx by Myriad Genetic Laboratories, Inc.; PD-L1 IHC 22C3 pharmDx by Dako North America, Inc.; and Foundation One CDx by Foundation Medicine, Inc., play a crucial role in this process. Exact Sciences presented novel long-term patient results in multi-cancer early detection and breast cancer recurrence testing at the American Society of Clinical Oncology (ASCO) 2023. The analysis of the SEER program reaffirms the continued confidence in the prognostic value of the Oncotype DX Breast Recurrence Score 3. Moreover, the study also highlighted the test's prognostic capability for breast cancer-specific mortality in patients with invasive ductal carcinoma and invasive lobular breast cancer.
End Use Insights
The hospitals and clinics segment is expected to demonstrate a steady growth rate of 7.68% due to the rising number of hospitalizations and increasing burden of breast cancer. These healthcare facilities play a critical role in conducting biopsy procedures after the initial screening tests, while advanced imaging technologies such as PET, CT, & MRI are utilized for effective disease monitoring and treatment evaluation. Hospitals and clinics often have multidisciplinary teams, including oncologists, radiologists, pathologists, surgeons, and nurses, who collaborate to provide comprehensive care for breast cancer patients. This integrated approach ensures that patients receive appropriate diagnostic evaluations, treatment planning, and ongoing management.
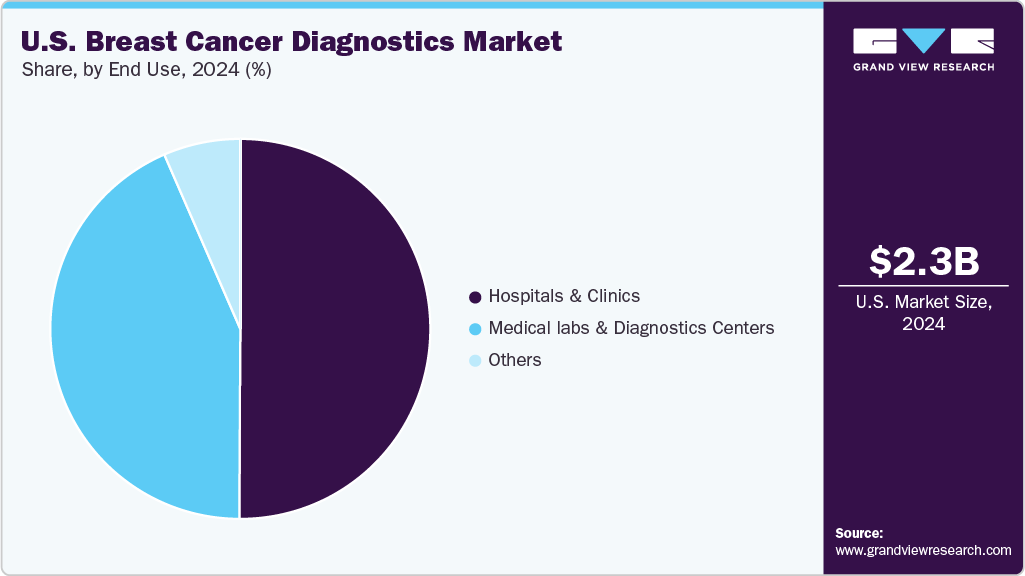
However, the medical labs & diagnostics centers segment is expected to witness the highest CAGR, owing to high market penetration and procedure volumes. An increase in the number of initiatives undertaken by governments to provide various services, such as reimbursement for diagnostic tests, is expected to boost market growth. Many healthcare institutions are working with laboratories to integrate different tests, such as mammography, ultrasound, and MRI.
In January 2025, The Laredo Medical Center recently inaugurated its Women's Imaging Center, aiming to enhance access to mammograms, diagnostics, and biopsies for women. In previous years, only 40% of women received mammograms due to scheduled backlogs or limited accessibility. This new center seeks to address these challenges by offering advanced diagnostic services, including breast ultrasounds, biopsies, and bone densitometry.
Key U.S. Breast Cancer Diagnostics Company Insights
Key players operating in the U.S. breast cancer diagnostics market are undertaking various initiatives to strengthen their presence and increase the reach of their products and services. Strategies such as expansion activities and partnerships are key in propelling the market growth.
Key U.S. Breast Cancer Diagnostics Companies:
- Hologic Inc.
- Genomic Health (Exact Sciences Corporation)
- BD
- Danaher
- Koninklijke Philips N.V.
- QIAGEN
- Thermo Fisher Scientific Inc.
- Argon Medical Devices, Inc.
- Myriad Genetics
- F. Hoffmann-La Roche Ltd.
Recent Developments
-
In January 2025, Roche's PATHWAY HER2 (4B5) test received FDA approval to identify HER2-ultralow metastatic breast cancer patients eligible for ENHERTU, expanding treatment options. This advancement builds on the HER2- low classification introduced in 2022, offering a new avenue for targeted therapy in patients with minimal HER2 expression.
-
In February 2024, Hologic received FDA clearance for its Genius Digital Diagnostics System, the first digital cytology platform combining AI and volumetric imaging to enhance cervical cancer screening accuracy. The system reduces false negatives by 28% and enables remote case reviews, improving lab efficiency and patient care.
-
In September 2024, Hologic responds to the recently issued breast cancer screening guidelines by the U.S. Preventive Services Task Force (USPSTF). The company provides a statement acknowledging the importance of early detection and emphasizing the continued need for regular mammography screenings in reducing breast cancer mortality rates.
-
In October 2024, Myriad Genetics launched five research collaborations to evaluate the use of its Precise MRD test for molecular residual disease (MRD) detection in breast cancer. These studies aim to enhance early recurrence detection, guide treatment decisions, and improve monitoring by measuring circulating tumor DNA (ctDNA) levels.
U.S. Breast Cancer Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 2.41 billion
Revenue forecast in 2033
USD 4.53 billion
Growth rate
CAGR of 8.21% from 2025 to 2033
Base year for estimation
2024
Historical data
2021 - 2023
Forecast period
2025 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, product, application, end use
Country scope
U.S.
Key companies profiled
Hologic Inc.; Genomic Health (Exact Sciences Corporation); BD; Danaher; Koninklijke Philips N.V.; QIAGEN; Thermo Fisher Scientific Inc.; Myriad Genetics; Argon Medical Devices, Inc.; F. Hoffmann-La Roche Ltd.
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
U.S. Breast Cancer Diagnostics Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis of the latest trends in each of the sub-segments from 2021-2033. For this study, Grand View Research has segmented the U.S. breast cancer diagnostics market report by type, product, application, and end use:
-
Type Outlook (Revenue, USD Million, 2021 - 2033)
-
Imaging
-
Biopsy
-
Genomic Tests
-
Blood Tests
-
Others
-
-
Product Outlook (Revenue, USD Million, 2021 - 2033)
-
Platform-based products
-
Next Generation Sequencing
-
Microarrays
-
PCR
-
Others
-
-
Instrument-based products
-
Imaging
-
Biopsy
-
-
-
Application Outlook (Revenue, USD Million, 2021 - 2033)
-
Screening
-
Diagnostic & Predictive
-
Prognostic
-
Research
-
-
End Use Outlook (Revenue, USD Million, 2021 - 2033)
-
Hospitals & Clinics
-
Medical labs & Diagnostics Centers
-
Others
-
Frequently Asked Questions About This Report
b. The U.S. breast cancer diagnostics market size was estimated at USD 2.26 billion in 2024 and is expected to reach USD 2.41 billion in 2025.
b. The U.S. breast cancer diagnostics market is expected to witness a compound annual growth rate of 8.21% from 2025 to 2033 to reach USD 4.53 billion in 2033.
b. The instrument-based segment held the largest share of 70.64% in 2024 due to government initiatives to promote screening and favorable reimbursement scenarios for diagnostic and screening tests.
b. Some key players operating in the U.S. breast cancer diagnostics market include Hologic, Inc.; Exact Sciences Corporation; Becton Dickinson, and Company; Koninklijke Philips N.V.; Qiagen; and Myriad Genetics.
b. The growth can be attributed to the increasing prevalence of breast cancer and rising government initiatives to increase the screening & diagnostic rate. For instance, according to the American Cancer Society, Breast cancer remains the most prevalent cancer among women in the U.S., excluding skin cancers, accounting for nearly 30% of all new female cancer cases annually. In 2025, an estimated 316,950 new cases of invasive breast cancer and 59,080 cases of ductal carcinoma in situ (DCIS) will be diagnosed. Tragically, 42,170 women are expected to die from the disease. The increasing incidence of breast cancer fuels demand for advanced diagnostic technologies such as mammography, genetic testing, and AI-powered imaging. Rising awareness, government initiatives, and expanding insurance coverage further stimulate the adoption of screening programs and innovative diagnostics across U.S.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










