- Home
- »
- Clinical Diagnostics
- »
-
Monocyte Activation Test Market Size, Industry Report, 2030GVR Report cover
![Monocyte Activation Test Market Size, Share & Trends Report]()
Monocyte Activation Test Market (2025 - 2030) Size, Share & Trends Analysis Report By Product (MAT Kits, Reagents), By Source (PBMC Based, Cell Line Based), By Application, By End-use, By Region, And Segment Forecasts
- Report ID: GVR-4-68040-510-0
- Number of Report Pages: 180
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Monocyte Activation Test Market Summary
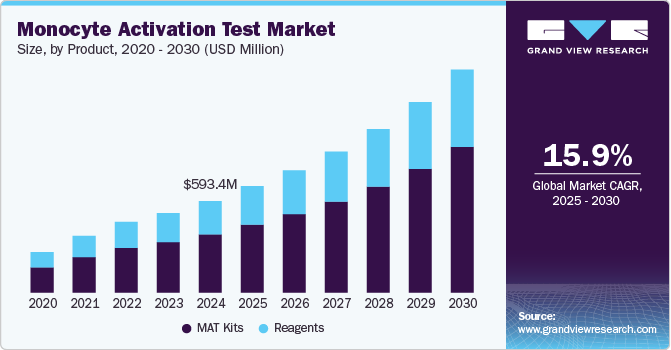
The global monocyte activation test market size was estimated at USD 593.4 million in 2024 and is projected to reach USD 1,445.8 million by 2030, growing at a CAGR of 15.92% from 2025 to 2030. The market is driven by the growing need for safer pharmaceutical products and the rising adoption of in-vitro alternatives to animal testing.
Key Market Trends & Insights
- In terms of region, North America was the largest revenue generating market in 2024.
- The U.S. monocyte activation test (MAT) market is experiencing robust growth.
- By product, the MAT Kits segment held the largest share of 63.7% in 2024.
- By source, the PBMC based segment held the largest share of 62.7% in 2024.
- By application, the drug development-based segment held the largest share of 40.6% in 2024.
Market Size & Forecast
- 2024 Market Size: USD 593.4 Million
- 2030 Projected Market Size: USD 1,445.8 Million
- CAGR (2025-2030): 15.92%
- North America: Largest market in 2024
Regulatory support, including endorsements by organizations like the FDA and European Pharmacopeia, has boosted monocyte activation test (MAT) adoption. Increasing awareness about ethical testing methods and advancements in MAT technology are notable trends. The market is also influenced by the rising prevalence of chronic diseases and biologics development, necessitating accurate pyrogen detection. Technological innovations and collaborations further enhance market growth, addressing industry demand for reliability and precision.
The Monocyte Activation Test (MAT) has emerged as a dependable, sustainable, and animal-free method for detecting both endotoxin and non-endotoxin pyrogens (NEPs). It is validated to identify pyrogenic contaminants by activating human monocytes, which release endogenous mediators like IL-6. Compared to traditional methods such as the Rabbit Pyrogen Test (RPT), MAT offers a more ethical and efficient alternative for pyrogen detection. Regulatory support, including its inclusion in the European Pharmacopoeia (Ph. Eur. 2.6.30) and ongoing efforts to phase out RPT, has significantly boosted its adoption in pharmaceutical and medical device industries.
Conventional MAT assays employ Peripheral Blood Mononuclear Cells (PBMCs) and rely on ELISA readouts. However, these methods face challenges such as lot-to-lot variability, extended assay times, and labor-intensive processes. To overcome these limitations, innovative solutions like the LumiMAT™ assay have been developed. The LumiMAT system incorporates a luciferase reporter gene to detect activated NF-κB protein, enabling rapid and highly sensitive pyrogen detection. By eliminating the need for ELISA and serum-based components, LumiMAT reduces reaction times and ensures consistent results. These advancements are accelerating the adoption of MAT in industries that require precise and efficient pyrogen detection methods.
The European Pharmacopoeia Commission (EPC) has taken significant steps to eliminate the Rabbit Pyrogen Test (RPT) by July 2025, mandating the use of in vitro methods like MAT for pyrogen testing. This transition aligns with sustainability goals and reflects industry trends toward more ethical testing practices. MAT’s ability to assess human inflammatory responses ensures the safety, efficacy, and quality of drugs, vaccines, and medical devices, making it a preferred choice for manufacturers. The updated guidelines emphasize risk-based assessments and standardized methodologies, facilitating MAT’s integration into routine quality control processes.
Innovative systems like the PyroCell MAT System by Lonza Bioscience are transforming the MAT market. Developed in partnership with Sanquin Reagents B.V., this system uses cryopreserved PBMCs, eliminating the need for donor qualification and cell isolation. The ready-to-use PyroCell system ensures on-demand availability, supporting pharmaceutical and biologics manufacturers in product development and release. It is particularly effective for detecting pyrogens in complex formulations like vaccines and cell-based biologics. By providing sensitive, reliable pyrogen detection without the use of experimental animals, PyroCell aligns with sustainability objectives and reinforces MAT’s role as a leading solution for pyrogen testing.
Market Concentration & Characteristics
The market is characterized by moderate-to-high levels of innovation, including the frequent development and introduction of novel techniques and diagnostic technologies like point-of-care diagnostics kits, which define the global monocyte activation test (MAT) market. Key players are investing in innovative methods and technologies to meet the global demand of the market.
The market is characterized by the leading players with moderate levels of product launches and merger and acquisition (M&A) activity. Market players like Merck KGaA, Thermo Fisher Scientific, Charles River Laboratories International, Inc., Sanquin are involved in new product launches and mergers and acquisitions. Such strategic activities as M&A, partnership, and collaboration are only serving to increase company’s competitiveness and expand their geographic reach and help to enter new territories.
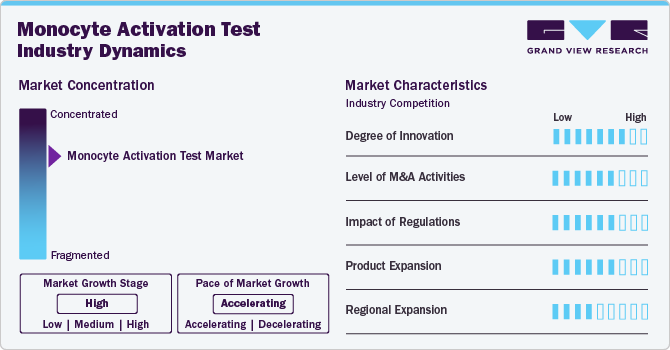
The Monocyte Activation Test (MAT) market demonstrates a high degree of innovation, driven by advancements in in-vitro pyrogen detection methods. Cutting-edge technologies like the LumiMAT™ assay have introduced luciferase reporter genes to enhance sensitivity and reduce assay times compared to traditional ELISA-based MAT systems. For instance, innovations such as cryopreserved PBMCs in systems like Lonza's PyroCell MAT have addressed challenges of donor variability and cell isolation, ensuring consistency and scalability. Regulatory shifts, including the phasing out of the Rabbit Pyrogen Test (RPT), have further accelerated the development of novel MAT solutions. These innovations position MAT as a sustainable, precise, and efficient alternative for pyrogen detection.
The Monocyte Activation Test (MAT) market is witnessing a moderate level of mergers and acquisitions (M&A) activity, primarily driven by the growing demand for sustainable and animal-free pyrogen testing solutions. Key players in the pharmaceutical and diagnostics sectors are pursuing strategic acquisitions to expand their portfolios with innovative MAT technologies. Collaborations, such as Lonza Bioscience's partnership with Sanquin Reagents B.V. for the PyroCell MAT system, highlight the trend of combining expertise to develop advanced solutions. M&A activities are also focused on integrating proprietary technologies, enhancing distribution networks, and meeting regulatory requirements, fostering growth and innovation within the MAT market.
Regulations play a pivotal role in shaping the Monocyte Activation Test (MAT) market, driving its adoption as an ethical and sustainable alternative to traditional pyrogen testing methods like the Rabbit Pyrogen Test (RPT). The inclusion of MAT in the European Pharmacopoeia (Ph. Eur. 2.6.30) and the European Pharmacopoeia Commission’s (EPC) decision to phase out RPT by 2025 have significantly boosted MAT's market potential. These regulatory shifts align with global sustainability goals and emphasize in-vitro testing methods. Compliance requirements for pharmaceutical and medical device manufacturers to ensure product safety and quality further accelerate MAT integration, fostering market growth and innovation.
In the Monocyte Activation Test (MAT) market, product substitutes primarily include traditional pyrogen testing methods such as the Rabbit Pyrogen Test (RPT) and the Limulus Amebocyte Lysate (LAL) test. While RPT has been widely used, it faces ethical concerns and regulatory restrictions due to its reliance on animal testing. The LAL test, derived from horseshoe crab blood, is limited to detecting endotoxins and lacks the capability to identify non-endotoxin pyrogens (NEPs). These limitations position MAT as a superior alternative, offering a more comprehensive, ethical, and sustainable solution for pyrogen detection, which reduces the viability of traditional substitutes in the evolving market.
The Monocyte Activation Test (MAT) market is experiencing significant geographical expansion, driven by increasing regulatory support and demand for ethical pyrogen testing solutions. North America and Europe lead the market due to stringent safety regulations, advanced healthcare infrastructure, and the phasing out of animal-based tests like the Rabbit Pyrogen Test (RPT). Emerging markets in Asia-Pacific, particularly in countries like China and India, are witnessing rapid adoption due to growing pharmaceutical and biologics industries. Collaborative efforts among global players to establish distribution networks and regulatory alignment further enhance MAT accessibility. This expansion underscores MAT's global relevance in ensuring product safety and compliance.
Product Insights
The MAT Kits segment held the largest share of 63.7% in 2024, the MAT is a highly reliable in vitro test used to assess pyrogenicity, particularly in pharmaceutical and medical device testing. With increasing regulatory pressure for safer products, the MAT has become the gold standard for endotoxin testing, replacing older methods like the Limulus Amebocyte Lysate (LAL) test. One major driver is the growing demand for safety in biopharmaceuticals and medical devices. The MAT allows for a more comprehensive detection of pyrogens, including those that traditional endotoxin tests may not identify. This has led to its adoption in drug development and manufacturing processes, especially for biologics and vaccines. For example, the MAT is widely used in the testing of intravenous drugs and implantable devices, where safety is critical.
Additionally, the MAT’s ability to provide more accurate results in shorter timeframes compared to older methods is boosting its popularity in the market. As the need for rapid and precise testing grows, regulatory bodies and manufacturers are increasingly favored by MAT kits, cementing their dominant market share.
The reagent segment in the monocyte activation test (MAT) market is witnessing the fastest growth rate. One major driver is the growing emphasis on the development and production of biologics, where MAT reagents are crucial in meeting the safety standards set by regulatory bodies such as the FDA and EMA. For example, MAT reagents are used to test intravenous drugs, vaccines, and implantable devices, where pyrogenicity must be thoroughly assessed. Additionally, the MAT's ability to offer faster and more reliable results compared to older methods, such as the Limulus Amebocyte Lysate (LAL) test, is contributing to its increasing adoption, further strengthening the demand for MAT reagents in the market.
Source Insights
The PBMC based segment held the largest share of 62.7% in 2024. The PBMC-based MAT is a more advanced and sensitive method of pyrogen testing, as it uses human immune cells to mimic the human immune response better, providing more accurate results compared to traditional animal-based tests. One major driver is the increasing demand for more reliable and human-relevant testing methods. PBMC-based MAT offers a more physiologically relevant model by utilizing human cells, which are more predictive of human responses to pyrogens. This is particularly important in the testing of biologics and vaccines, where safety is paramount. For example, PBMC-based MAT is being used to develop and test new biologic drugs to ensure their safety before clinical trials.
Another driver is the growing regulatory pressure to replace animal testing with in vitro methods. The PBMC-based MAT aligns with this shift, as it reduces the need for animal models while maintaining high accuracy in pyrogen detection. This regulatory push, along with advancements in cell culture technologies, is further propelling the adoption of PBMC-based MAT in pharmaceutical and medical device testing.
The cell line based segment market is experiencing significant growth. This method uses immortalized human cell lines, such as THP-1 or U937 cells, to simulate the human immune response, providing a reliable and cost-effective alternative to traditional pyrogen testing methods. A primary driver is the growing demand for more reproducible and standardized testing methods. Cell line-based MAT offers consistency in results since it uses immortalized cell lines, reducing the variability that can occur with primary human cells or animal models. This consistency is crucial for regulatory compliance in industries like pharmaceuticals and medical devices, where safety standards must be met rigorously.
Application Insights
The drug development-based segment held the largest share of 40.6% in 2024. The use of the Monocyte Activation Test (MAT) in drug development is being driven by several key factors, making it an essential tool in ensuring the safety of new drugs, particularly biologics and vaccines. One primary driver is the increasing regulatory demand for more reliable and human-relevant pyrogen testing methods. Regulatory agencies like the FDA and EMA are increasingly favoring in vitro tests like MAT over traditional animal-based methods for endotoxin testing, as MAT provides a more accurate reflection of human immune responses.
In drug development, especially for biologics, ensuring the safety of products before clinical trials is crucial. MAT is used to detect pyrogens, including endotoxins and non-endotoxins, which can cause fever and other adverse reactions in patients. For example, MAT is used extensively in the testing of monoclonal antibodies and vaccines to ensure they do not induce harmful immune responses. Additionally, MAT’s ability to provide faster results compared to older methods, like the Limulus Amebocyte Lysate (LAL) test, is a significant advantage in the fast-paced drug development process. This speed helps pharmaceutical companies streamline their testing and reduce time-to-market for new therapies.
The vaccine development segment market is experiencing significant growth. In vaccine development, the Monocyte Activation Test (MAT) plays a crucial role in ensuring safety by detecting pyrogens that could cause adverse reactions such as fever. One key driver is the increasing regulatory focus on non-animal testing methods. MAT, using human immune cells, offers a more accurate and ethical alternative to animal-based pyrogen testing. For example, MAT is employed to test vaccines like those for COVID-19, where safety is paramount. The test’s ability to rapidly detect both endotoxins and non-endotoxins helps ensure that vaccines are safe for human use before clinical trials, meeting stringent regulatory standards.
End-use Insights
The pharmaceutical industry segment held the largest market share of 56.3% in 2024. In the pharmaceutical industry, the Monocyte Activation Test (MAT) is becoming increasingly essential for ensuring the safety of drugs, particularly biologics and vaccines. The growing regulatory emphasis on replacing animal testing with more human-relevant, in vitro methods is a major driver. MAT, which uses human immune cells, provides more accurate and reproducible results compared to traditional animal-based endotoxin testing. For example, MAT is used to test monoclonal antibodies and intravenous drugs, ensuring they do not cause harmful immune responses like fever. As the pharmaceutical industry continues to prioritize safety and compliance with global regulations, MAT is becoming the preferred method for pyrogen testing, helping speed up drug development while ensuring product safety.
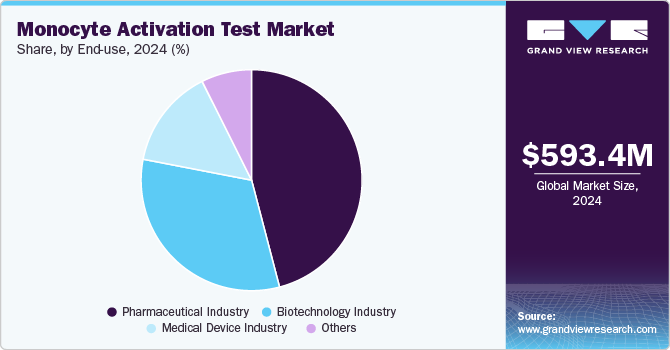
In the biotechnology industry, the Monocyte Activation Test (MAT) is a critical tool for ensuring the safety of biologic products, including gene therapies, monoclonal antibodies, and vaccines. A key driver for MAT adoption is the increasing regulatory demand for reliable, human-relevant testing methods. MAT, by using human immune cells, provides a more accurate and predictive assessment of pyrogenicity compared to traditional animal testing, aligning with the biotechnology industry's focus on innovation and ethical practices. For example, MAT is used in the testing of cell and gene therapies to ensure they do not induce harmful immune responses in patients. As biotechnology companies develop more complex biologics, the need for sensitive and rapid pyrogen testing becomes crucial. MAT helps meet these needs by offering faster results, which accelerates the development timeline and ensures that products meet the safety standards required by regulatory bodies like the FDA and EMA.
Regional Insights
North America monocyte activation test market dominated with a share of 36.65% in 2024, driven by the presence of well-established pharmaceutical and medical device industries. The region's focus on patient safety and quality control has further fueled MAT adoption. Regulatory acceptance by organizations such as the United States Pharmacopeia (USP) and the Government of Canada has supported MAT integration into industry practices.
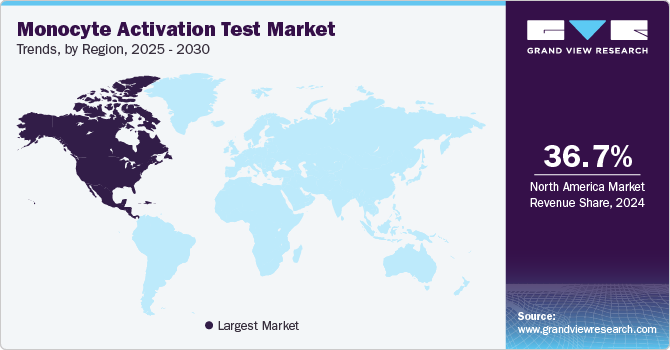
U.S. Monocyte Activation Test Market Trends
The U.S. monocyte activation test (MAT) market is experiencing robust growth. The country's robust pharmaceutical and biotechnology sectors, coupled with stringent regulatory standards, have driven the demand for reliable pyrogen testing methods like MAT. The U.S. Food and Drug Administration (FDA) recognizes MAT as an acceptable alternative to traditional endotoxin testing, further promoting its use.
Europe Monocyte Activation Test Market Trends
Europe is a key region for MAT adoption, with countries like Germany, the United Kingdom, and France leading the market. The European Medicines Agency (EMA) has endorsed MAT as a valid method for pyrogen testing, aligning with the region's commitment to patient safety and ethical testing practices. The market is expected to continue its growth trajectory, driven by advancements in biotechnology and pharmaceutical research.
UK monocyte activation test (MAT) market seen a rise in MAT utilization, particularly in vaccine development and medical device testing. Regulatory bodies like the Medicines and Healthcare products Regulatory Agency (MHRA) support the use of MAT, aligning with the UK's commitment to ethical and reliable testing methods.
Monocyte activation test (MAT) market in France is increasingly adopting MAT, driven by the need for accurate and humane pyrogen testing methods. The French Medicines Agency (ANSM) recognizes MAT as a valid alternative to traditional testing, promoting its integration into industry practices.
Germany monocyte activation test (MAT) Market is growing contribution of pharmaceutical industry in the MAT market, which is showcasing a large adoption of in vitro testing methods. The country's emphasis on innovation and regulatory compliance has led to a growing preference for MAT in drug development and manufacturing processes.
Asia Pacific Monocyte Activation Test Market Trends
The Asia Pacific Monocyte Activation Test (MAT) market is experiencing significant growth, driven by the region’s expanding pharmaceutical and biotechnology industries. Countries like China, Japan, India, and South Korea are at the forefront of adopting MAT due to increasing regulatory demands for reliable and humane pyrogen testing methods. MAT, which uses human immune cells for pyrogen detection, aligns with the region’s growing focus on ethical and efficient testing.
The increasing prevalence of chronic diseases and the rise in biologic drug development are key factors contributing to the demand for MAT in the region. For example, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) supports the use of MAT, and China’s National Medical Products Administration (NMPA) recognizes its importance in regulatory practices. Moreover, the growing emphasis on safety and quality control in drug manufacturing and vaccine development further accelerates MAT adoption.
China monocyte activation test (MAT) market is growing due to rapid adoption of MAT by pharmaceutical industry, driven by the need for reliable and efficient pyrogen testing methods. Regulatory bodies are increasingly recognizing MAT as a valid alternative to traditional testing, promoting its integration into industry practices.
Monocyte activation test (MAT) market in Japan is growing due to their commitment to patient safety and ethical testing practices has led to a growing preference for MAT in drug development and manufacturing. The Pharmaceuticals and Medical Devices Agency (PMDA) supports the use of MAT, aligning with Japan's regulatory standards.
Latin America Monocyte Activation Test Market Trends
Latin America is witnessing a gradual increase in MAT adoption, with countries like Brazil leading the market. The region's growing pharmaceutical and biotechnology sectors are driving the demand for reliable pyrogen testing methods. Regulatory bodies are beginning to recognize MAT as a valid alternative to traditional testing, promoting its integration into industry practices.
Brazil monocyte activation test (MAT) market is increasingly adopting MAT by pharma industries, driven by the need for accurate and humane pyrogen testing methods. The Brazilian Health Regulatory Agency (ANVISA) is beginning to recognize MAT as a valid alternative to traditional testing, promoting its integration into industry practices.
Middle East & Africa Monocyte Activation Test Market Trends
The Middle East and Africa region is gradually adopting MAT, with countries like Saudi Arabia leading the market. The region's growing healthcare infrastructure and emphasis on patient safety are key drivers of this trend. Regulatory bodies are beginning to recognize MAT as a valid alternative to traditional testing, promoting its integration into industry practices.
Saudi Arabia monocyte activation test (MAT) market is driven by the need for reliable and efficient pyrogen testing methods. The Saudi Food and Drug Authority (SFDA) is beginning to recognize MAT as a valid alternative to traditional testing, promoting its integration into industry practices.
Key Monocyte Activation Test Company Insights
Some of the key market players include Lonza Group, Charles River Laboratories, Bio-Rad Laboratories, Merck KGaA, Seikagaku Corporation. These players are undertaking various strategic initiatives to increase their share in the market. New product development, collaborations, and partnerships are some such endeavors.
Key Monocyte Activation Test Companies:
The following are the leading companies in the monocyte activation test market. These companies collectively hold the largest market share and dictate industry trends.
- Lonza Group
- Charles River Laboratories
- Bio-Rad Laboratories
- Merck KGaA
- Seikagaku Corporation
- Hyglos GmbH
- Wako Chemicals USA
- Thermo Fisher Scientific
- MAT BioTech
- Eurofins Scientific
Monocyte Activation Test Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 690.65 million
Revenue Forecast in 2030
USD 1,445.84 million
Growth rate
CAGR of 15.9% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in (USD Million) and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product; source; application; end-use; region
Region scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Lonza Group; Charles River Laboratories; Bio-Rad Laboratories; Merck KGaA; Seikagaku Corporation; Hyglos GmbH; Wako Chemicals USA; Thermo Fisher Scientific; MAT BioTech; Eurofins Scientific
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Monocyte Activation Test Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented global monocyte activation test market based on product, source, application, end-use, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
MAT Kits
-
Reagents
-
-
Source Outlook (Revenue, USD Million, 2018 - 2030)
-
PBMC Based
-
Cell Line Based
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Drug Development
-
Vaccine Development
-
Medical Device Testing
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharmaceutical Industry
-
Biotechnology Industry
-
Medical Device Industry
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
Kuwait
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global monocyte activation test market size was estimated at USD 593.40 million in 2024 and is expected to reach USD 690.65 million in 2025.
b. The global monocyte activation test market is expected to grow at a compound annual growth rate of 15.92% from 2025 to 2030 to reach USD 1,445.84 million by 2030.
b. North America dominated the market with a share of 36.65% in 2024, driven by the presence of well-established pharmaceutical and medical device industries. The region's focus on patient safety and quality control has further fueled MAT adoption. Regulatory acceptance by organizations such as the United States Pharmacopeia (USP) and the Government of Canada has supported MAT integration into industry practices.
b. Some key players operating in the monocyte activation test (MAT) market include Lonza Group, Charles River Laboratories, Bio-Rad Laboratories, Merck KGaA, Seikagaku Corporation, Hyglos GmbH, Wako Chemicals USA, Thermo Fisher Scientific, MAT BioTech, Eurofins Scientific
b. The monocyte activation test (MAT) market is driven by the growing need for safer pharmaceutical products and the rising adoption of in-vitro alternatives to animal testing. Regulatory support, including endorsements by organizations like the FDA and European Pharmacopeia, has boosted MAT adoption. Increasing awareness about ethical testing methods and advancements in MAT technology are notable trends. The market is also influenced by the rising prevalence of chronic diseases and biologics development, necessitating accurate pyrogen detection. Technological innovations and collaborations further enhance market growth, addressing industry demand for reliability and precision.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










