- Home
- »
- Biotechnology
- »
-
U.S. Genome Editing Market Size, Industry Report, 2030GVR Report cover
![U.S. Genome Editing Market Size, Share & Trends Report]()
U.S. Genome Editing Market (2024 - 2030) Size, Share & Trends Analysis Report By Technology (CRISPR, ZFN), By Delivery Method (Ex-vivo, In-vivo), By Mode (Contract, In-house), By Application, By End-use, And Segment Forecasts
- Report ID: GVR-4-68040-245-3
- Number of Report Pages: 80
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
U.S. Genome Editing Market Size & Trends
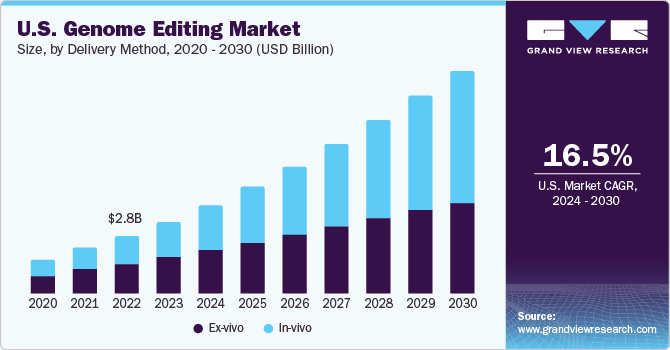
The U.S. genome editing market size was estimated at USD 3.49 billion in 2023 and is expected to grow at a CAGR of 16.5% from 2024 to 2030. The growth in the genome editing market is driven by rising demand for synthetic genes, the application of CRISPR technology, and government funding. The expansion is further fueled by the production of genetically modified crops, genomics projects, and advancements in research and development. Innovative gene editing methods have advanced gene therapy and molecular biology significantly. The availability of user-friendly gene therapy systems and the potential for rapid genome-wide analyses also contribute to the market growth.
The U.S. accounted for over 43.9% of the global genome editing market in 2023. The advent of innovative technologies enabling easy editing of genomic DNA has unlocked a transformative period in therapeutic development. Genome editing technology has the potential to take precision medicine to the next level. Anticipated regulatory approvals for research and clinical trials involving gene editing therapies will likely boost their acceptance soon. Successful outcomes in preclinical and phase I clinical trials are projected to impact product introductions and stimulate market growth with promising opportunities.
In recent years, the market has expanded primarily due to the rising demand for synthetic genes and the increased use of CRISPR genome editing technology in various biotech sectors. The growth is expected to be propelled by increased government funding, a surge in the production of genetically modified crops, and a rise in genomics projects. CRISPR-based diagnostic tools, instrumental in COVID-19 diagnostics, also drive this growth. For example, the VaNGuard (Variant Nucleotide Guard) diagnostic test, developed by academics from Nanyang Technological University in March 2021, can identify mutated SARS-CoV-2 strains, thereby enhancing the use of CRISPR genome editing technology in the diagnostics sector.
Furthermore, ongoing advancements in gene-editing technologies significantly contribute to the market’s profitability. For instance, scientists at Harvard’s Wyss Institute for Biologically Inspired Engineering developed a new gene-editing tool called Retron Library Recombineering (RLR) in April 2021. This tool enables the execution of millions of genetic experiments simultaneously, thereby enhancing editing rates. The market expansion is driven by supportive government policies concerning synthetic biology, a growing demand for engineered genes and cells, and increased government and major corporations investments.
Developing and applying novel gene-editing techniques have marked significant progress in gene therapy and molecular biology, further propelling the market’s growth. In addition, the market growth during the projected period is fueled by the availability of easy-to-use gene therapy systems, progress in genome engineering, and the ability of gene therapy to expedite comprehensive analyses of gene function across the genome. Although gene editing technologies are confined to the basic research area in drug development, they hold great potential for detecting and validating new therapeutic targets. It also plays a great role in understanding a drug’s mechanism of action by providing a broader picture of genes involved in regulating various cell biological processes.
Market Concentration & Characteristics
The U.S. genome editing industry is fragmented, with numerous entities playing a part. Leading global firms such as Thermo Fisher Scientific, Inc. and GenScript have a significant presence in this market. The industry also has a variety of key players, demonstrating a multifaceted environment with many important contributors. The industry’s fragmented nature is further emphasized by various companies specializing in genome editing technologies, each of which plays a crucial role in propelling therapeutic applications and research initiatives. Companies in the U.S. genome editing sector are deeply engaged in product innovation, mergers, acquisitions, and regional expansion to strengthen their market presence.

Recent technological innovations are driving significant advancements in research, leading to the development of novel products and therapies. These innovations are revolutionizing the field by enabling scientists to explore cutting-edge gene editing applications. Moreover, changes in regulatory policies governing gene editing in the U.S. create a conducive environment that encourages researchers to move into new and innovative uses of gene editing technology, further propelling the growth.
Mergers and acquisitions play a crucial role in the industry by facilitating strategic partnerships, technology licensing, and enhancing capabilities. These corporate activities enable companies to expand their footprint, access new technologies, and drive innovation in gene editing. Mergers and acquisitions in the genome editing sector foster growth and drive advancements that shape the future of genetic engineering. For instance, in February 2023, Ensoma acquired Twelve Bio, a gene editing company, to enhance genomic medicine capabilities through CRISPR technology and in vivo treatments.
The regulatory paradigm followed for gene editing is distributed among the coordinated framework of the Department of Agriculture (USDA), Environmental Protection Agency (EPA), and the US Food and Drug Administration (FDA) rather than on biosafety law. This framework is significantly product-based, and the process comes into the picture for regulatory assessment. Under strict conditions, the permission to carry out gene editing for human therapeutic applications can be a benchmark in the gene editing market. For instance, in July 2023, Excision BioTherapeutics publicized that the FDA granted their CRISPR-based treatment, EBT-101, Fast Track Designation.
The rising competition in the market is evident through the growing number of products entering clinical trials or progressing toward preclinical research. This influx highlights the industry's dynamic nature, with companies actively developing and advancing genetic therapies to address various diseases, fostering innovation and progress in genome editing technologies.
By expanding geographically, companies can benefit from local expertise, establish strategic partnerships, and cater to specific regional needs. This expansion fosters innovation, drives industry growth, and enhances competitiveness by adapting products and services to meet the demands of different regions within the industry, ultimately leading to increased share and sustained profitability.
End-use Insights
Biotechnology and pharmaceutical companies dominated the market and accounted for the largest revenue share of 50.8% in 2023 due to the increasing number of research activities aimed at developing new therapies. Furthermore, collaborations between pharmaceutical companies and emerging firms to innovate new technologies are prevalent. The surge in research and development efforts for new treatments is a key driver of revenue growth. In addition, the market is expected to grow due to strategic developments by major companies. For instance, in January 2022, CRISPR Therapeutics AG and Capsida Biotherapeutics Inc. entered a strategic agreement to develop innovative gene therapies using CRISPR/Cas9 technology to treat various diseases. Per the agreement, Capsida will provide its adeno-associated virus (AAV) delivery technology, while CRISPR Therapeutics will lend its genome editing expertise.
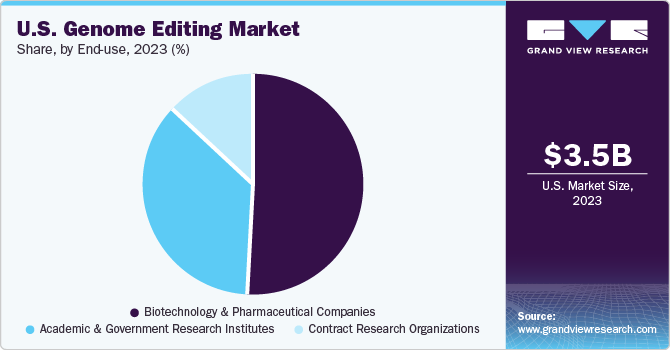
Academic and research institutions are expected to grow at the fastest CAGR of 18.8% during the forecast period due to the growing adoption of technology in universities. Numerous organizations are creating educational materials for high school and college students to facilitate their understanding of gene editing processes. For instance, in January 2022, the ChristianaCare Gene Editing Institute visited high schools and colleges with its innovative CRISP in Box Educational Toolkit. This toolkit aims to raise awareness about powerful biomedical technologies like CRISPR, which are on the verge of revolutionizing disease treatment. The current kit, which only contains safe, synthetic components, cannot be used to modify living organisms. However, it serves as an effective tool to illustrate how CRISPR works.
Delivery Method Insights
Ex-vivo dominated the market and accounted for the largest revenue share of 50.8% in 2023 due to its benefits, such as ease of control in DNA alteration. The growth of the ex-vivo delivery segment has been fueled by an expanding clinical trial pipeline that utilizes genome editing tools. The successful completion of the Human Genome Project (HGP) has led to the discovery of nearly 7,000 new human genetic diseases. Gene therapies based on ex vivo gene editing, like CAR-T, have significantly impacted. However, most patients with genetic diseases need in vivo treatments. These treatments require clinically precise editing tools and safe, targeted delivery systems that can be scaled up to serve the entire patient population. This need is a contributing factor to the growth of these segments.
In-vivo segment is expected to grow at the fastest CAGR of 19.4% during the forecast period. The in-vivo method, which involves gene editing within the body, can treat many diseases as some cells cannot be removed and restored. In-vivo gene editing allows delivery of the nucleases by viral vector, nonviral vector, or direct delivery of nucleases protein. For instance, systemic therapy targets liver tissues to treat hemophilia, while local injections target specific tissues like muscle, brain, or eye. Many cells cannot survive outside the body or be effectively returned post-treatment, so most diseases require in-vivo genome editing.
Technology Insights
CRISPR/Cas9 dominated the market and held the largest revenue share of 41.8% in 2023 and is expected to grow at the fastest CAGR during the forecast period. This technique, which can precisely split DNA strands and introduce new genetic data, is invaluable in gene therapy, drug development, and scientific research. CRISPR/Cas9 is favored for genome editing due to its simplicity, high efficiency, and accuracy, making it versatile for various applications. Its clinical applications have gained widespread acceptance, as demonstrated by the increasing number of ongoing clinical trials that employ gene-editing techniques to treat a broad spectrum of diseases, including AIDS, cancer, and genetic disorders. Beyond human health, this technology is also seeing growing adoption in fields like agriculture and animal breeding.
TALENs held the second-largest market share during the base year. The DNA-binding protein of TALEN technology can be engineered to identify specific DNA sequences, enabling accurate gene modification. This technique has many applications, including gene therapy, agricultural enhancement, and disease modeling. The shortcomings of CRISPR technology have led to an increase in the use of the TALEN method in certain challenging genomic regions to edit. A study released in January 2021 found TALEN to be a more appropriate tool for compact DNA studies. These types of studies are expected to drive the use of the TALEN method in research environments in the future.
Mode Insights
Contract dominated the market with the largest revenue share of 65.5% in 2023 and is expected to grow at the fastest CAGR during the forecast period. This is due to the extensive outsourcing in the gene editing field, which offers lower costs and more operational flexibility than in-house development. The segment’s growth is expected to continue, driven by the expanding capabilities of major players in this area. For example, in December 2022, a Contract Research Organization (CRO) called Crown Bioscience, Inc. partnered with ERS Genomics Limited to access ERS’s CRISPR/Cas9 patent portfolio, enhancing its position in the gene editing market. Such initiatives will likely increase outsourcing opportunities in the genome editing sector, positively impacting market growth.
In-house is projected to experience significant growth in the upcoming years, owing to the benefits of in-house operations. These benefits include control over supply channels, improved abilities to troubleshoot processes, and the potential for scaling up in-house activities in the future, which could be advantageous in the long term. Consequently, companies like Precision BioSciences maintain their cGMP-compliant manufacturing facilities that generate genome-edited products, such as allogeneic CAR T cell therapies. These elements are expected to contribute to the segment’s revenue growth during the forecast period.
Application Insights
Genetic engineering dominated the market and held the highest revenue share of 68.4% in 2023 and is expected to grow at the fastest CAGR during the forecast period. This is attributed to the fast-paced advancements in gene and stem cell therapy. CRISPR gene editing’s application in human induced pluripotent stem cells (hiPSCs) has significant implications for treating various diseases. Cell line engineering, a technique that alters cell genetics to modify or create organism traits, is widely used in biotechnology, gene therapy, and drug development research. It holds the potential to revolutionize medicine. The cell line engineering market is poised for rapid expansion due to technological progress and increasing demand for customized medical treatments.
Clinical application is expected to grow at the second fastest rate of CAGR 12.8% during the forecast period. The efficiency of CHO cell lines, often used in large-molecule medicine production, is being enhanced by CRISPR technology. This has propelled the biopharmaceutical industry forward, boosting the genome editing market. Genome editing technologies and genetic engineering hold substantial growth potential in medical areas such as diagnostics and drug development. For example, the effectiveness and safety of UCART123 are being evaluated in a clinical trial funded by Cellectis S.A. for patients with relapsed/refractory acute myeloid leukemia using TALEN gene-editing technology. The application of genome editing in drug discovery and development is expected to significantly increase due to the growing demand for innovative, powerful treatments for various diseases. Genome editing can create new medicines that target genes or gene pathways, potentially leading to more efficient and specialized disease treatments.
Key U.S. Genome Editing Company Insights
Companies in the U.S. genome editing industry are entering into licensing agreements with technology developers to strengthen their presence in the market. Key industry participants actively pursue strategic actions such as acquisitions, partnerships, and collaborations to optimize their market share. For instance, in April 2022, Thermo Fisher Scientific introduced the Gibco CTS TrueCut Cas9 Protein, a top-tier support material for researchers transitioning from basic research to therapeutic applications utilizing genome editing techniques. This product shows promise for studying CAR T-cell therapies through CRISPR-Cas9 genome editing, consistently demonstrating over 90% efficacy in human primary T-cells and high editing efficiency across various cell lines.
Key U.S. Genome Editing Companies:
- Merck KGaA
- Cibus
- Recombinetics, Inc.
- Sangamo
- Editas Medicine
- Precision Biosciences
- CRISPR Therapeutics
- Intellia Therapeutics, Inc.
- Caribou Biosciences, Inc.
- Cellectis S.A.
- GenScript
- AstraZeneca
- Integrated DNA Technologies, Inc.
- Egenesis Inc.
- New England Biolabs
- OriGene Technologies, Inc.
- Lonza
- Thermo Fisher Scientific, Inc.
Recent Developments
-
In February 2024, Precision BioSciences, Inc. (Nasdaq: DTIL), specializing in advanced gene editing, uses its unique ARCUS platform to create in-vivo treatments. These treatments involve complex gene modifications, including insertion, removal, and deletion.
-
In November 2023, AstraZeneca Holding B.V. and Cellectis initiated a collaborative research project. This collaboration aims to utilize Cellectis' gene editing and production expertise to expedite the creation of advanced treatments. These treatments are primarily targeted towards areas with a significant lack of adequate solutions, such as cancer, immunology, and rare diseases.
-
In January 2023, Editas Medicine finalized a contract with Shoreline Biosciences, as per which Shoreline Biosciences takes over Editas Medicine's preclinical programs. These programs involve gene-edited induced pluripotent stem cells (iPSC) that are developed into natural killer cells (iNK), including the EDIT-202 program. The agreement also includes the transfer of related manufacturing technologies.
U.S. Genome Editing Market Report Scope
Report Attribute
Details
Revenue forecast in 2030
USD 10.69 billion
Growth rate
CAGR of 16.5% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Technology, delivery method, mode, application, end-use
Country scope
U.S.
Key companies profiled
Merck KGaA; Cibus; Recombinetics, Inc.; Sangamo; Editas Medicine; Precision Biosciences; CRISPR Therapeutics; Intellia Therapeutics, Inc.; Caribou Biosciences, Inc.; Cellectis S.A.; GenScript; AstraZeneca; Integrated DNA Technologies, Inc.; Egenesis Inc.; New England Biolabs; OriGene Technologies, Inc.; Lonza; Thermo Fisher Scientific, Inc.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
U.S. Genome Editing Market Report Segmentation
This report forecasts revenue growth in the U.S. market and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the U.S. genome editing market based on technology, delivery method, mode, application, and end-use:
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
Meganucleases
-
(CRISPR)/Cas9
-
TALENs/MegaTALs
-
ZFN
-
Others
-
-
Delivery Method Outlook (Revenue, USD Million, 2018 - 2030)
-
Ex-vivo
-
In-vivo
-
-
Mode Outlook (Revenue, USD Million, 2018 - 2030)
-
Contract
-
In-house
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Genetic Engineering
-
Cell Line Engineering
-
Animal Genetic Engineering
-
Plant Genetic Engineering
-
Others
-
Clinical Applications
-
Diagnostics
-
Therapy Development
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Biotechnology & Pharmaceutical Companies
-
Academic & Government Research Institutes
-
Contract Research Organizations
-
Frequently Asked Questions About This Report
b. The U.S. genome editing market size was estimated at USD 3.49 billion in 2023.
b. The U.S. genome editing market is expected to grow at a compound annual growth rate (CAGR) of 16.5% from 2024 to 2030 to reach USD 10.69 billion by 2030.
b. Ex-vivo dominated the market and accounted for the largest revenue share of 50.8% in 2023 due to its benefits, such as ease of control in DNA alteration.
b. Some prominent companies in the U.S. genome editing market include Merck KGaA; Cibus; Recombinetics, Inc.; Sangamo; Editas Medicine; Precision Biosciences; CRISPR Therapeutics; Intellia Therapeutics, Inc.; Caribou Biosciences, Inc.; Cellectis S.A.; GenScript; AstraZeneca; Integrated DNA Technologies, Inc.; Egenesis Inc.; New England Biolabs; OriGene Technologies, Inc.; Lonza; Thermo Fisher Scientific, Inc.
b. The growth in the genome editing market is driven by rising demand for synthetic genes, the application of CRISPR technology, and government funding.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










